Support Page Content
Battling Opiate Overdoses
By Rodney Sime
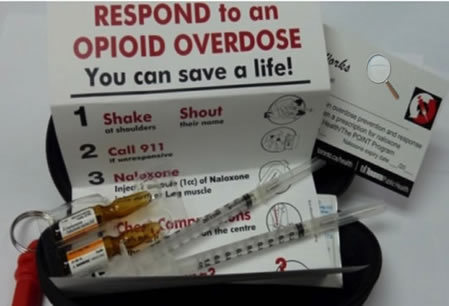
Recently I read that Walgreen, CVS and some other pharmacies have placed the drug Naloxone on an over-the-counter status: no prescription required. Naloxone has no pharmacological properties of its own but it is a narcotic antagonist that powerfully counteracts the euphoria, respiratory depression, nausea, convulsions, and other effects produced by a variety of opiate narcotics. Morphine, heroine and other narcotic agonists, act by attaching to receptors in the brain. Naloxone, whose molecular structure is very similar to morphine, attaches itself even more strongly than morphine to the brain receptors, quickly displacing morphine. When given to a comatose addict, the patient recovers consciousness within minutes, can speak rationally, and is out of danger. Compact Naloxone rescue kits are now available to first responders and others (Figure 1).
I first heard of Naloxone in 1973. I was a young professor of chemistry looking for my next research project. By then the drug problem was well underway, and it occurred to me that the molecular structure of certain narcotics might be of real interest. I asked Dr. Suzanne A. Snively, director of the CSUS Student Health Services, if she could suggest any opiates whose structures would be of importance. (Today a scholarship in her name memorializes her many years dedicated to student health.) Dr. Snively immediately suggested Naloxone, oxymorphone, and nalbuphine. I wrote to the DuPont Chemical Company describing my research plans, and requested samples of Naloxone and the other two drugs. They promptly sent 100 mg. samples of the three drugs by return mail. They required no background check, no forms, nothing, even though oxymorphone and nalbuphine are narcotic agonists about 100 times more powerful than morphine. Access and movement of narcotics is much more highly regulated nowadays.
Events coalesced rapidly. UC Berkeley had recently acquired a new CAD – 4 X-ray diffractometer and donated their old GE XRD-5 to us. I drove a CSUS truck to Berkeley, loaded the diffractometer on, drove back, and installed it in the basement of Sequoia Hall. A newly arrived grad student, Richard Forehand, had expressed an interest in X-ray crystallography. Together we set up the diffractometer, mounted a single crystal of Naloxone in its goniometer and began measuring the intensities of X-rays diffracted by the Naloxone crystal. After Richard had measured nearly 1500 diffraction intensities, we began the process of solving the structure. While these activities were going on, I sent a grant proposal to the National Institutes of Health describing my project to solve the crystal and molecular structures of Naloxone (Figure 2), oxymorphone, and nalbuphine. The proposal was granted and I spent a 1974-1975 sabbatical at the Swiss Institute of Technology in Zurich. At Zurich computers much more powerful than ours permitted a more thorough refinement of the structure.
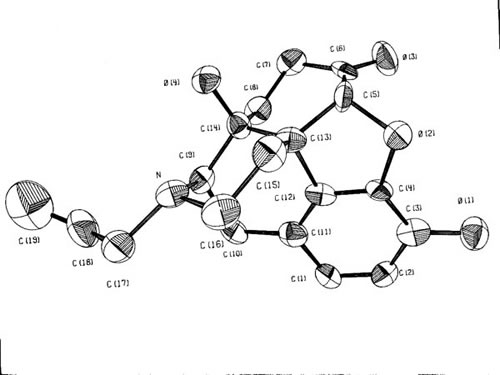
(For clarity, the hydrogen atoms have been omitted.)
We published three papers on these three opioids in Acta Crystallagraphica, an internationally read journal. Listed below the authors’ names is the line: “Contribution from the Chemistry Department, California State Sacramento, California.” It reflects well on our university when its name appears in a prestigious international research journal. Richard Forehand was a co-author on the first, the Naloxone paper. His contribution was invaluable. You can read the entire paper by googling “Crystal and Molecular Structure of Naloxone.