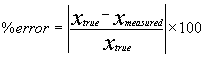This is the Procedures file. The data sheets will be distributed in
lab.
This is a Word 6.0 file. If you have
difficulty downloading the Word file (*.doc) files, or want to use a
word processing program other than Word, try downloading the rich
text format (*.rtf) version below. You may find it necessary to save
the file first, and then open the word processing program.
Download:
lab2proc.rtf
Return to Index
CE 170: Water Quality and Supply Engineering
Lab 2: Use of Laboratory
Equipment -- Accuracy and Precision
Purpose of Lab
The purpose of this lab is two-fold:
- To become familiar with the use of selected laboratory
equipment.
- To examine the concepts of precision and accuracy as they
relate to laboratory procedures used in environmental engineering.
Statistical tools will be used to evaluate precision and accuracy.
Definitions
Precision: "Precision refers to the
reproducibility of a method when it is repeated on a homogeneous
sample under controlled conditions, regardless of whether or not the
observed values are widely displaced from the true value as a result
of systematic or constant errors present throughout the measurements.
Precision can be expressed by the standard deviation."
(Standard Methods)
Accuracy: "Accuracy refers to the
agreement between the amount of a component measured by the test
method and the amount actually present." (Standard Methods)
Mean, X: 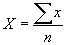
where:
x - measurement value
n - number of times measurement was made
Sample Standard Deviation, s :
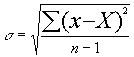
Coefficient of Variation (percentage),
COV: 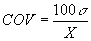
95% Confidence Interval: 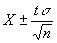
where:
t = 1.96 when n is greater than 10
Relative Error, % error: 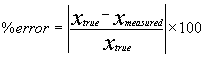
Procedures - Mass and Volume
Measurements
A. Measurement of Mass:
Equipment: Analytical balance
- Measure the mass of an index card provided by the instructor
using the analytical balance. Each group should do this
independently of other groups. Be careful to not touch the index
card with your hands because this may transfer oils to the card
and change its mass.
- When all groups have weighed the index card, exchange data
with other groups. Have one member of each group sign the index
card.
- Repeat the first step. Exchange that data with the other
groups.
- Determine the mean, sample standard deviation, and coefficient
of variation for the two sets of mass measurements (both before
and after the card was signed). Record your results and comment on
the precision of the analytical balance on attached sheets. Show a
sample calculation on separate sheet(s). In this sample
calculation, write out all the equations for the mean, standard
deviation, and coefficient of variation.
B. Measurement of Volume
Equipment:
one 10 mL graduated (Mohr) pipette, marked "TD" - to deliver
one 50 mL graduated cylinder, marked "TD" - to deliver
one 100 mL volumetric flasks, marked "TC" - to contain
six 50 mL beakers
Platform balance
- Note and record the water temperature. This temperature will
be used to calculate the true volume from the true weight.
- Measure and record the mass of the 50 mL beakers. Be sure to
keep them straight.
- Pipette 8.4 ml of water from the supply provided by the
instructor into the one of the 50 mL beakers. Follow your
instructor's directions on how to pipette 8.4 mL from a graduated
pipette. Using the analytical balance, measure and record the mass
of the 50 mL beaker and water. Repeat the process twice more using
a new 50 mL beaker each time. Each person in the lab group should
do the measurement at least once.
- Place 44 mL of water into the 50 mL graduated cylinder. Pour
that water into a fresh 50 mL beaker. Using the analytical
balance, measure and record the mass of the 50 mL beaker and
water. Repeat the process twice more using a new 50 mL beaker each
time. Each person in the lab group should do the measurement at
least once.
- Weigh and record the mass of the 100 mL volumetric flask using
the platform balance. Weigh and record the mass of the 100 mL
volumetric flask and water. Repeat the process twice more using
the original dry tare weight.
- Exchange your volume measurement data with the other groups in
the lab.
- Determine the mean, standard deviation, coefficient of
variation, and 95% confidence interval for each volume
measurement. Provide sample calculations. In this case, you may
use you calculator's statistical functions for mean and standard
deviation (i.e., you don't have to write out the equations).
Note: When using a pipette and volumetric flask, the liquid
will have a surface which is curved slightly downward. This curved
surface is called the meniscus, and the bottom of the meniscus should
be exactly on the line indicating the desired beginning or end of the
dispensation of liquid. This is true for any graduated cylinder,
burette, etc.
Discussion Questions
- Comment on the precision of each volume measurement.
- Compare the accuracy of each type of volume measurement.
- How large a role does the lab technicians technique play for
these type of volume measurements?
![]()
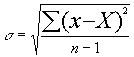
![]()
![]()