Presentations: 9:15 a.m. – 10:35 a.m.
Session 1: Physical and Computational Chemistry Location: Foothill
Suite, Auburn Room (3rd floor)
Session Chair: Dr.
Benjamin Gherman (California State University,
Sacramento)
Session 2: Biochemistry I Location: Orchard
Suite (2nd floor)
Session Chair: Dr.
Katherine McReynolds (California State University,
Sacramento)
Session 3: Biochemistry II Location: Forest Suite (2nd floor)
Session Chair: Dr. Tom Savage (California
State University,
Sacramento)
|
9:15 a.m.
|
Trinh Thai, Andrew J. Clifford, Hosea L. Matel
University
of California, Davis
Research Advisor:
Dr. Krishnan Nambiar
|
9.
Bioconversion of
α-Ionone to Hydroxy α-Ionone by a Streptomyces Strain
|
|
9:35 a.m.
|
Imad Sawaya, Hosea Matel, Andrew J. Clifford,
Daniela Hampel, Susan Ebeler
University
of California, Davis
Research Advisor:
Dr. Krishnan Nambiar
|
10.
Production of Zeaxanthin
from Recombinant E. coli
|
|
9:55 a.m.
|
Thomas Gildea, Hosea Matel
University
of California, Davis
Research Advisor:
Dr. Krishnan Nambiar
|
11.
Synthesis of
9’-Carboxychromanol, a Metabolite of Vitamin E
|
|
10:15 a.m.
|
Jonathan Grist, Heather Wright
San Jose
State University
Research Advisor:
Dr. Elaine D. Collins
|
12.
Synthesis and Purification
of TAR RNA and Tat Peptides from Bovine Immunodeficiency Virus
|
Session 4: Organic and Atmospheric Chemistry Location: Foothill
Suite, Folsom Room (3rd floor)
Session Chair: Dr.
James Miranda (California State University,
Sacramento)
List of Presentation Abstracts
1. Computational Study of the Cyclization Reactions of
Naphthyl-Substituted Enediynes. 2
Nadezhda Korovina, Benjamin F.
Gherman*, John D. Spence*
California
State University, Sacramento, Department of Chemistry
2. Prediction of Reduction
Potentials from Electron Affinities for Metal-Salens and Application to the
Reduction of Alkyl Halides 2
Victor A. Mendiola, Kris
A. Pineda, Benjamin F. Gherman*, and James A. Miranda*
California
State University, Sacramento, Department of Chemistry
3. Theoretical Hydration
Energies of Biologically Relevant α-Keto Amides. 2
Henry Wedler, Christian
S. Hamann, Dean J. Tantillo*
University
of California, Davis, Department of Chemistry
4. The pKas and pH dependent
lumiscence of N-(2-methylbutyl)-4-aminodipicolinic acid (MEBADPA) 2
Andrew J. Ingram,
Alexander G. Dunlap, Gilles Muller*
San
Jose State University, Department of Chemistry
5. Gel Encapsulation
Techniques of Corallina vancouverensis Acetone Powder (CVAP) containing the
Enzyme Bromoperoxidase 2
John Kim, Roy Okuda*
San
Jose State University, Department of Chemistry
6. Determination of Binding
Association of Mastoparan X to Liposomes
by Isothermal Titration Calorimetry 2
Frankie Robert Gonzales,
Jennifer Pomponio, Kaitlin Fisher, Jennifer Lillig*
Sonoma
State University, Department of Chemistry
7. The Chemical Biology of
Hydrogen Sulfide: Generation of
Persulfides and Biological Implications. 2
Samantha J. Carrington,
Adam Dudik, Heather Turner, Nestor Francoleon, Jon M. Fukuto*
Sonoma
State University, Department of Chemistry
8. Probing the Effects of
Promoter Concentration on Yield in the One-Pot Synthesis of a-linked Glycolipids. 2
Francine E. Park,
Matthew Schombs, Suvarn S. Kulkarni, Jacquelyn Gervay-Hague*
University
of California, Davis, Department of Chemistry
9. Bioconversion of
α-Ionone to Hydroxy α-Ionone by a Streptomyces Strain. 2
Trinh Thai, Andrew J.
Clifford, Hosea L. Matel, Krishnan Nambiar*
University
of California, Davis, Department of Chemistry
10. Production of Zeaxanthin
from Recombinant E. coli 2
Imad Sawaya, Hosea Matel,
Andrew J. Clifford, Daniela Hampel, Susan Ebeler, Krishnan P. Nambiar*
University
of California, Davis, Department of Chemistry
11. Synthesis of
9’-Carboxychromanol, a Metabolite of Vitamin E.. 2
Thomas Gildea, Hosea
Matel, Krishnan Nambiar*
University
of California, Davis, Department of Chemistry
12. Synthesis and Purification
of TAR RNA and Tat Peptides from Bovine Immunodeficiency Virus. 2
Jonathan Grist, Heather
Wright, Elaine D. Collins*
San
Jose State University, Department of Chemistry
13. A Novel Ether Synthesis in
Ionic Liquids. 2
Seham Afaghani, Beth
Kochly*
Mills
College, Department of Chemistry
14. Synthesis of sub-micron
poly(N-isoproopyl acrylamide) gel/oil capsules using polymerize-to method. 2
Lauren E. Tracy, Conor
P. Roche, Antje Echterhoff, Thorsteinn Adalsteinsson*
Santa
Clara University, Department of Chemistry and Biochemistry
15. The effect of tethered
dendrimer size on density and reactivity of immobilized metal chelate indicator
displacement assays 2
Michelle Nguyen, Matthew
Greaney, Cheng-Chi Chang, Lawrence Margerum*
University
of San Francisco, Department of Chemistry
16. PEPICO Studies on the
Energetics of Atmospherically Relevant SxOyClz
Ions. 2
Lauren Ooka, Sampada
Borkar, Andras Bodi, Thomas Gerber, Bálint Sztáray*
University
of the Pacific, Department of Chemistry
(Note: In each
abstract, the presenting author is underlined and the faculty mentor(s) is
denoted with an asterisk.)
1.
Computational Study of the Cyclization Reactions of
Naphthyl-Substituted Enediynes
California State
University, Sacramento, Department of Chemistry
Enediyne compounds,
which upon Bergman cyclization produce diradical species, can be utilized as
pro-drugs for DNA cleavage. The
cyclization reaction can be initiated thermally or photochemically to produce
the diradical. By introducing naphthyl
substituents on the alkyne termini, the wavelength of light necessary to induce
photocyclization may be extended into the near-UV region. Increased steric bulk, however, may allow
direct C1-C5 cyclization to become competitive with C1-C6
Bergman cyclization. Computational
methods applied to the thermal cyclization reaction have been used to
understand the relative tendencies of C1-C5 versus C1-C6
cyclization. By appending
electron-withdrawing and electron-donating groups to the naphthyl substituents,
the electronics can be modulated to further affect the wavelength of light
needed for photocyclization as well as the preference for the competing
cyclization pathways. Using density
functional theory (DFT) calculations, the kinetics and thermodynamics of the C1-C5
and C1-C6 cyclization reactions have been computed for
enediynes with variously positioned and substituted naphthyl substituents under
a range of reaction conditions.
Time-dependent DFT calculations have been used to compute UV-Vis spectra
for the enediynes. Results indicate that
the favorability of the C1-C5 reaction increases through
the inclusion of electron-withdrawing and electron-donating groups, whereas by
strategic positioning of the naphthyl steric bulk, the preference for C1-C6
cyclization increases. In addition,
while the absorption λmax for the enediyne is not greatly
affected by electron-donating groups on or repositioning of the naphthyl
substituents, electron-withdrawing groups can potentially appreciably alter the
absorption λmax. These
results suggest the ability to tune the cyclization reactivity of
naphthyl-substituted enediynes.

California State
University, Sacramento, Department of Chemistry
Reduced nickel(II)-
and cobalt(II)-salen compounds can catalytically reduce alkyl halides. These reactions have environmental
significance, as effective reduction of halogenated organic substrates
facilitates the conversion of harmful herbicides and fungicides to more benign
materials. The two processes involved here
– reduction of metal(II)-salens and reaction of reduced metal-salens with alkyl
halides – have yet to have their thermodynamics studied in detail. Computational chemistry can be used to obtain
this information for not only nickel(II)- and cobalt(II)-salen, but for a wide
range of other metal-salens. First, in
order to establish a means of predicting reduction potentials for metal-salen
compounds, electron affinities (EAs) for a training set comprised of 19 metal-salens,
containing a variety of metal centers and electron-donating and
electron-withdrawing substituents, are computed using density functional theory
(DFT). Concurrently, Epc
values are measured for the metal-salens using cyclic voltammetry following
two-step syntheses. Epc for
the training set metal-salens are effectively correlated to the computed EAs,
forming a basis for predicting reduction potentials of new test sets of
metal-salens. Second, the thermodynamics
of the reaction of reduced forms of metal-salens from both the training and test
sets with a variety of alkyl halides is determined using additional DFT
calculations. Free energy changes are
computed for reactions along pathways leading back to the metal(II)-salen plus
an alkyl radical and the halide ion or leading to alkylation of the imino bond
in the salen plus release of the halide ion.
Results indicate that the thermodynamics of dehalogenation can vary
appreciably with the identity of the metal-salen.

University of California, Davis, Department of Chemistry
α-Keto amides
are used in the pharmaceutical industry. Two drug molecules which contain
α-keto amide functionality are Varespladib, a drug used for treating acute
coronary syndrome, and Boceprevir, a drug used for treating hepatitis C. Our
goal is to use hydration propensity as a lens through which we can predict what
form of the α-keto amides is more active, i.e., the ketone or the ketone
hydrate. All hydration energies were calculated using the B3LYP method using
the 6-31+G(d,p) basis set using the Gaussian 03 suite.
In this
investigation, we calculated the energies of hydration of numerous α-keto
amides in vacuum. Next, these α-keto amides were optimized using water and
chloroform as solvents. These solvent calculations employed the same method and
basis set as above. Both UAKS and UA0
radii were investigated for all solvent calculations. Their energies of
hydration were determined in both solvents.
Thus far, analysis of the hydration reaction in vacuum suggests that the
ketone form is favored for all α-keto amides. Aside from a few
fluorine-containing compounds, this trend holds in water and chloroform using
UAKS radii. However, when using UA0 radii many compounds both in water and in
chloroform favored the hydrate.
Ultimately, our
goal is to use hydration energy to investigate what form of these compounds is
most active in the human body and hence what form (ketone or hydrate) should be
expected in α-keto amide-containing pharmaceuticals.
San
Jose State University, Department of Chemistry
The effect of pH on
the structure and luminescence properties of MEBADPA have been determined. The pKas of the molecule were determined by
spectrophotometric titration using a custom peristaltic flow system to carry
the titration solution to the spectrometer.
In order to examine the luminescence of each of these species, the
quantum yield of this molecule was measured as a function of pH. This data showed that the luminescence
increases as MEBADPA is fully deprotonated, but then shuts down as the
concentration of hydroxide increases.
After a series of control experiments as well as luminescence, pH, and
temperature studies, we have concluded that fully deprotonated MEBADPA is
collisionally quenched by free hydroxide in solution via the Stern-Volmer
model. This has allowed the luminescence
spectra to be corrected for quenching and an absolute quantum yield for MEBADPA
to be determined. Using the pKas and
quenching constant the intensity has been modelled as a function of pH in order
to determine the optimum pH for luminescence.
San
Jose State University, Department of Chemistry
Gel encapsulation is
a method that allows for the reuse of enzymes.
Ideal encapsulation methods form matrixes that allow for the passage of
substrates and products through the gel, but keeps the enzyme entrapped within
the gel. In this project, sodium
alginate and tetramethyl orthosilicate (TMOS) are used to encapsulate Corallina
vancouverensis acetone powder (CVAP), which contains a bromoperoxidase. The encapsulation and re-use of the gels were
verified qualitatively and semi-quantitatively using a colormetric phenol red
assay. Both the sodium alginate and TMOS
gels were found to successfully encapsulate CVAP and allow for the re-use of
the bromoperoxidase enzyme.
Sonoma State
University, Department of Chemistry
Background: This
project focuses on studying the binding association of the anti-microbial
peptide mastoparan X (MPX) to liposomes by isothermal titration calorimetry
(ITC). MPX, found in wasp venom, is
known to associate with lipid membranes.
To better understand the features of binding, ITC is being used to
determine an apparent association constant.
Knowledge of this affinity will help to determine how MPX targets
cells. These experiments will provide
the foundation for studying the binding of anti-listerial bacteriocins to
membranes by ITC. Listeria, a bacteria
found in raw meats and processed foods, can cause many illnesses, including
fetal miscarriages.
Methods: Mastoparan
X peptide was purchased from Bachem. DMPC (dimyristoylphosphatidylcholine) and
DMPG (dimyristoylphosphatidylglycerol) were purchased from Avanti and liposomes
were prepared with varying compositions of each lipid. Sonication provided small unilamellar
vesicles (SUVs) and extrusion provided large unilamellar vesicles (LUVs). The total heat of complete peptide binding
was determined by titrating the peptide solution into a solution of excess
liposomes and the binding isotherm was found by titrating the peptide solution
with a liposome suspension. The
background was determined by titrating liposomes into buffer without peptide,
and buffer without liposomes into a peptide solution. Background was subtracted from the
experimental data and analyzed using Origin 7.0 software.
Results: Binding has successfully been measured
between MPX and DMPC:DMPG liposomes (1:1) by ITC. The total enthalpy of binding was found to be
-7.35 kcal/mol, suggestive of an exothermic binding event. The molar ratio of binding was found to be 89
liposome sites per peptide site. The
association constant was determined to be 8.76E5 M-1. Reproduction of this data was
successful. Titrations with zwitterionic
liposomes yielded less binding.
Conclusions: Results suggest that binding of MPX is
stronger to anionic liposomes than to zwitterionic liposomes. This is possibly due to the electrostatic
interaction between the positively charged peptide and the negatively charged
liposomes. Further studies are currently
being conducted to determine the association with liposomes of varied
composition and in the presence of varied salt concentration.
Sonoma State
University, Department of Chemistry
Hydrogen sulfide (H2S)
has been reported to be an endogenously generated signaling molecule of
significant physiological importance.
For example, it can elicit smooth muscle relaxation and inotropy and can
protect against ischemia-reperfusion injury.
In spite of its important functions, the chemistry by which it acts is
unknown. Considering that H2S
is a simple and potentially reactive thiol, it seems reasonable to speculate
that its chemical biology will include redox reactions with other biological
thiols. Thus, the study herein examines
the redox chemistry of H2S with thiols and thiol proteins. Specifically, the reaction of H2S
with oxidized thiol species to form persulfide (RSSH) intermediates is
examined. We speculate that protein
persulfide formation can impart significant and important changes in protein
function. Moreover, we are developing
methods for the facile generation of biological persulfides as a means of
studying the effect this novel sulfur oxidation state can have on protein
function.
University of California, Davis, Department of Chemistry
In 1993, the first a-linked
glycolipids were discovered from marine sponge.
Subsequent SAR studies culminated with the development of a-linked
galactosylceramide (KRN7000), which was found to elicit a significant
immunological response in murine models.
This created a need for access to diverse and anomerically pure sources
of a-linked glycolipids to further investigate the immunological
effects of these antigens. Although
synthetic approaches for making cerebrosides were available, a more efficient
model was desired. A major breakthrough
came in 2007 when the Gervay-Hague Laboratory published an efficient protocol
for the stereoselective, one-pot synthesis of a-linked
glycolipids. Building on the success of
their first generation process, the efficiency of the method lies in the
thoughtful combination of glycosyl iodide donors, tetrabutylammonium iodide and
careful selection of protecting groups.
This second generation technology facilitates the use of fully
functionalized ceramides. An area of
study that had not been explored was the effect that catalyst concentration has
on the stereochemical outcome of the glycosylation event. Described herein are the results of this
study. Although the optimal TBAI
concentration varies slightly, our findings can be applied to a wide variety of
a-linked
glycolipids, producing a consistent methodology for the synthesis of several
biologically applicable glycoconjugates.
University of California, Davis, Department of Chemistry
The conversion of
α-Ionone to hydroxy α-Ionone by an identified strain of Streptomyces
was investigated at different culturing temperatures, length of time of
culturing and initial pH of the culture medium. Conversion was higher at higher
temperature. The bioconversion time for α-Ionone can thus be shortened by
culturing the Streptomyces at higher temperature. But two other oxidation
metabolites are being produced at higher temperature. The initial pH of the
culture medium did not significantly affect conversion. The produced hydroxy
α-Ionone is a single enantiomer based on derivatization with MOSHER’s
chloride.
University of California, Davis, Department of Chemistry
Zeaxanthin is a
yellow pigment carotenoid produced by plants and algae. In an effort to
optimize the growth conditions for the production of zeaxanthin in recombinant E. coli engineered to overproduce this
compound, the effect of the initial pH of the culture medium was examined. The
E. coli were grown in Luria-Bertani (LB) broth containing ampicillin at 37°C at
initial pH of 7.1, 7.6, and 8.04. Cell density was measured by taking
absorbance at 660 nm at regular time intervals. The pH was also measured at
regular intervals. Plots of the
absorbance with respect to time revealed that the bacterium exhibited a
biphasic growth. Results showed that a very basic medium encourages much more
rapid initial growth (first phase) compared to neutral and slightly basic
media. The organism in neutral pH medium and in the slightly basic medium had
very similar initial growth rates. During the second phase, the organism in the
neutral pH medium had the fastest growth rate. Samples from each culture were
taken at different times, centrifuged to collect the cells, and extracted with
acetone. The acetone extracts were concentrated and the primary product was
isolated using HPLC on a C18 column using acetonitrile as the mobile phase. The
chromatograms showed a predominant peak at a retention time of 13.6 minutes.
The solution eluted at this time was collected and examined using NMR and mass
spectrometry. The spectral data showed that the compound formed was not
zeaxanthin. Because of the yellow color of the compound extracted from the
cells, it is possible that the compound initially produced was zeaxanthin and
underwent a reaction to give the final product. It is also possible that the
initial compound was an intermediate of zeaxanthin.
University of California, Davis, Department of Chemistry
Vitamin E is an
essential nutrient which has been implicated in maintaining good health. Besides its role as an antioxidant, little is
known about the physiological function of Vitamin E. As part of a human in vivo
study of how the body metabolizes RRR-a-Tocopherol, the primary form of vitamin
E absorbed by humans, it was necessary to synthesize its metabolites to use as
standards for analyzing samples. One of these metabolites is
9’-carboxychromanol. 9’-carboxychromanol
was synthesized using Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic
acid) and R-Citronellol (3,7-dimethyl-6-octen-1-ol) as starting materials.
Trolox was reduced with borane and the resulting alcohol oxidized to the
corresponding aldehyde after enzymatic resolution using lipase. R-Citronellol
was subjected to regioselective allylic carbon oxidation and the resulting
aldehyde protected as an acetal. Julia-Kocienski olefination reaction yields
the alkene. Subsequent acetal deprotection, catalytic hydrogenation and
oxidation of the aldehyde to the carboxylic acid yields 9’-carboxychromanol.
Details of the synthesis and structural characterization will be the subject of
this presentation.
San
Jose State University, Department of Chemistry
Bovine
immunodeficiency virus (BIV) is an advantageous model system for studying human
immunodeficiency virus (HIV) due to its similar structure and mechanism. The key
to pathogenicity is the interaction between the transactivator response element
(TAR) RNA and the trans-activating (Tat) peptide in both human and bovine
infections. The interaction of the Tat
protein and TAR RNA during the HIV life cycle causes viral RNA to transcribe
more efficiently and accumulate in the nucleus of its host. We have cloned the cDNA for TAR RNA with a
ribozyme, hepatitis delta virsus (HDV) ribozyme, and restriction sites through
polymerase chain reaction (PCR)-assisted gene assembly. The cDNA was subcloned into the expression
vector pUC-18 with the BamHI and HindIII restriction sites. The construct includes the cDNA for the
hepatitis delta virus (HDV) RNA,a self-cleaving ribozyme at the 3’ end of the
TAR sequence. This allows the production
of homogenous TAR RNA once transcription occurs whereas T7 RNA polymerase can
add 0, +1, +2 or +3 nucleotides to the 3’ end of the TAR RNA transcript. The ribozyme cleaves as the RNA is
synthesized, and the TAR RNA is purified from a low-melting point agarose
gel. We have also used PCR-assisted gene
assembly to clone cDNA for the wild-type Tat peptide cDNA, as well as the two
single mutants, R78G and K75G, and one double mutant, R78G/K75G. The Tat cDNA was subcloned into the
expression vector, pTwin-1 (NEB)
at the Pst I and Sap I restriction sites.
The Tat peptides were then expressed in E. coli ER2566 cells and
purified by affinity chromatography with chitin resin. Once each is produced,the interaction between
TAR RNA and Tat peptides will be observed using Isothermal Titration
Calorimetry (ITC). The goal is to
develop therapeutic agents that interfere with the interaction between TAR RNA
and Tat peptides.
Mills College, Department of Chemistry
It is the goal of
this research to perfect a synthetic method for preparing a variety of bulky
compounds by SN1 substitution in ionic liquids. Ionic liquids are a recyclable,
non-volatile, aprotic class of solvents. This novel synthetic method allows SN1
type reactions occur in an aprotic solvent, a rare reaction type. The reaction
under investigation is 1-adamantyl mesylate with methanol in
1-butyl-3-methylimidazolium bistriflamide (bmim NTf2). We have optimized
reaction conditions and NMR data shows substitution occurs in solvent mixtures
with as low as 13.73% methanol. Further research will explore the lowest
concentration of nucelophile required for a reaction to occur. In the future,
we hope to extend our research to other nucleophiles.
Santa
Clara University, Department of Chemistry and Biochemistry
Sub-micron sized
gel/oil capsules are synthesized using a polymerize-to method where a stable
mini-emulsion is used as a scaffold for deposition of an ultra-thin porous
gel-like polymer layer. Scaffolding, ranging between 100 and 200 nm is produced
via sonication of a water, sodium dodecyl sulfate (SDS) and long linear alkane
mixture. The polymer, N-isopropyl acrylamide, (PNIPAM), is deposited by direct
free radical synthesis that is initiated from the micellar surface using a
surface active initiator, tert-butylperoxy 2-ethylhexyl carbonate (tBEC). The
capsules have a gel-like polymer shell of controllable thickness between 20-50
nm. The polymerize-to method produces capsules that are thermally stable and we
have tested encapsulation of various types of oils, including octanol,
tetradecane and Isopar-M. The deposition of the polymer does not appear to
interfere with the composition of the oil phase, which may give us a pathway to
directly encapsulate fragile oil-soluble molecules within the sub-femtoliter
volume. These particles show significant potential for use as submicron
containers for targeted drug delivery.
University of San Francisco, Department of Chemistry
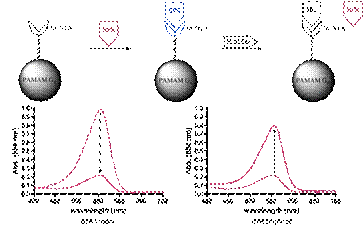
Surface tethered
dendrimers were used as platforms for colorimetric sensing of amino acids. Polyamidoamine dendrimers (PAMAM, Gx) were
covalently attached to porous beads and the resulting surface tethered
dendrimers were then modified with the metal chelating ligand
N,N-bis[carboxymethyl]-lysine (NTA). The
surface bound NTA’s were loaded with Ni2+ followed by exposure to
the metal indicator organic dye, bromopyrogallol red (BPR). Ternary complexes were formed between the
chelated metal ion and the dye as indicated by the beads slowly turning
blue. Indicator displacement assays were
carried out on 20 different amino acids to gain insight into the surface bound
dye release reaction. The Gx-NTA-Ni-BPR
surfaces show selectivity for histidine among the amino acids tested. Surface modification was varied using
dendrimer generations 3, 4 and 5 to gain insight into the effect of dendrimer
size and surface charge on the sensing ensemble. Preliminary results suggest that the larger
generations of dendrimers lead to a more sensitive and selective system.
Atmospheric sulfur
chemistry has immense significance to the biosphere. In order to model
interactions between the sulfur and halogen cycles in atmospheric models,
reliable thermochemical data is needed for the various SxOyClz
neutrals and ions. TPEPICO and iPEPICO experiments were performed on SCl2,
S2Cl2, SOCl2, and SO2Cl2
to derive the heats of formation of ions and their neutral counterparts to fill
this gap. The iPEPICO experiments were performed on the VUV beamline of the
Swiss Light Source. In dissociative photoionization, SCl2 leads to
SCl+, the heat of formation of which is determined. In the iPEPICO
experiment, S2Cl2 leads to S2Cl+
and then to S2+ in consecutive dissociation steps. In this case both the end
product and the neutral precursor have reliable literature heats of formation.
For SOCl2, the picture is similar, except that the thermochemistry
of SO+ is much better known and is not consistent with the heat of
formation of SOCl2 for which a more reliable number is given. The
heat of formation of SO2Cl+ is derived from the SO2Cl2
heat of formation. From our results, the sulfur-chlorine ionic bond energies
are measured. Also, since the four
systems are interconnected through a loss of oxygen, S=O bond energies are also
determined indirectly.
List of Poster Abstracts
1. Photoionization mass spectrometric studies of the
2-methyl-1-butanol + OH reaction. 2
Zhou Lu, David L.
Osborn, Craig A. Taatjes, and Giovanni Meloni*
a.
University of San Francisco, Department of Chemistry
b.
Combustion Research Facility, Sandia National Laboratories, Livermore, CA
2. Towards the synthesis of
fluorinated sialic acids. 2
Leon Castaneda, Laila
Dafik, Marc d'Alarcao*
San
Jose State University, Department of Chemistry
3. A review on the inhibitory
effect of ammonia on the photosynthetic oxygen evolution of the PSII protein
complex 2
Qi Mo, R. David Britt*
University of California, Davis, Department of Chemistry
4. Synthesis and
characterization of circularly polarized luminescence Ln(III)-containing probes. 2
Truman Jefferson, Andrew
J. Ingram, Eliseo Quiroz, Alex Dunlap, Gilles Muller*
San
Jose State University, Department of Chemistry
5. Adsorption and Phase Transition
of Dipalmitoylphosphatidylcholine Liposomes via Attenuated Total Reflectance
Fourier Transform Infrared Spectroscopy. 2
Mateo R.
Hernandez, Terry C.H. Ng, Brian
C. Walsh, Elyse N. Towns, Donald P. Land*
University of California, Davis, Department of Chemistry
6. Phosphorylation Effect of
S151A/E on Known Cardiomyopathy Mutations found in Human Cardiac Troponin I 2
Soyun Michelle Hwang,
Susan Nguyen, James Shackelford, Aldrin Gomes*
University
of California, Davis, Department of Biochemistry
7. A new hybrid enzyme for
the selective hydroxylation of substrate unactivated C-H bond using light and
water 2
Phuong Ngoc, Mary E.
Cooper, Ngoc Huynh, Misa Au, Lionel Cheruzel*
San
Jose State University, Department of Chemistry
8. Novel Molecular Imprinted
Polymer as P450 metalloenzyme active site mimic. 2
Ruby Lo, Matthew T.
Berry, Amandeep Nijjar, Lionel Cheruzel*
San
Jose State University, Department of Chemistry
9. A Study of Substituent
Effects on a Modified Ritter Reaction. 2
Daniel Vasilchuk,
Cynthia Kellen-Yuen*
California
State University, Sacramento, Department of Chemistry
10. Synthesis of Fe3O4/Polystyrene
Core-Shell Nanoparticles Using Atom Transfer Radical Polymerization. 2
Jake Abel, Steven
Farmer*
Sonoma
State University, Department of Chemistry
11. Efficient &
Environmentally Friendly Syntheses of Palladium Monomethyl Complexes. 2
Arturo Galano, Andrew
Bolig*
San
Francisco State University, Department of Chemistry & Biochemistry
12. Approaches to
regioselectivity in hetero-Diels-Alder reactions. 2
Erich Bowman, Jinsong
Zhang*
California
State University, Chico, Department of Chemistry
13. DFT Computational Study of
a Biomimetic Model for the Metalloenzyme Peptide Deformylase. 2
Darren Steele, Benjamin
Gherman*
California
State University, Sacramento, Department of Chemistry
14. Synthesis and Characterization
of Germanium-doped Silicotitanate Materials. 2
Corri L. Alexander,
Jacqueline Houston*
California
State University, Sacramento, Department of Chemistry
15. Effects of Ionic Strength
on Aqueous Solutions of Xanthene Dyes. 2
Nina Huynh, Deena
Sadeli, Silvio Rodriguez*
University
of the Pacific, Department of Chemistry
16. Computational Analysis does
not Support the Reported Synthesis of Dehydro[12]annulene. 2
Vivian Huynh, Taylor S. Wood, Claire
Castro*, William L. Karney*
University
of San Francisco, Department of Chemistry and Environmental Science
17. Synthesis of
Nucleobase-Calix[4]arene Conjugates via "Click" Chemistry. 2
Mikael Minier, Liang
Xue*
University
of the Pacific, Department of Chemistry
18. The Design and Synthesis of
Novel Nitroxyl (HNO) Donor Molecules. 2
Christopher L. Bianco,
Tyler A. Chavez, Jared Jackson, Jon M. Fukuto*
Sonoma
State University, Department of Chemistry
19. Synthesis and Testing of
Colloidal Nano-gels for Capillary Electrophoresis. 2
Shawn Khademi, Conor P.
Roche, Thorsteinn Adalsteinsson*
Santa
Clara University, Department of Chemistry and Biochemistry
20. Synthesis of hollow poly(N-tert-butyl
acrylamide) nano-capsules via meta-stable mini-emulsion droplet nucleation 2
Elena Zavala, Thorsteinn
Adalsteinsson*
Santa
Clara University, Department of Chemistry and Biochemistry
21. Progress Towards the Total
Synthesis of Gallicynoic Acids. 2
Ryan Barnes, Brandon Tautges,
Christina McCulley, David Ball*
California
State University, Chico, Department of Chemistry
22. Photochemical Studies of an
Iron-Only Hydrogenase. 2
Melinda Pope, Melanie
Lomotan, Haley King, Carmen Works*
Sonoma
State University, Department of Chemistry
23. Experimental and
Computational Studies of Bromocyclohexanone Systems. 2
Sandra Blair, David
Ball*, Randy Miller*
California
State University, Chico, Department of Chemistry and Biochemistry
24. Equilibrium Times and Partician
Coefficients of Sesquiterpene, Monoterpene, Wound, Isoprene and MBO Standard
Compounds Using Various Solid Phase Microextraction Fibers. 2
Niria Arellano-Jara,
Brad Baker*
California
State University, Sacramento, Department of Chemistry
25. Electroreductive
Cyclization of Methyl Cinnamate in the Presence of Nickel(II) Salen: A
Mechanistic Study 2
Kellie R. England, James
A. Miranda*
California
State University, Sacramento, Department of Chemistry
26. Determining the Rate of
Water Substitution in Trinuclear Bi-Oxocapped Carboxylate-Bridged Metal
Complexes Using 1H NMR Spectroscopy. 2
Kris Pineda, Jacqueline
Houston*
California
State University, Sacramento, Department of Chemistry
27. Tandem Cyclization
Reactions Utilizing 3-Amino-1,2,4-triazole. 2
Joel Rivera-Pallares,
Cynthia Kellen-Yuen*
California
State University, Sacramento, Department of Chemistry
28. Benzo-fused Twisted
[10]Annulenes. 2
Alyse Novoa, Claire
Castro*, William Karney*
University
of San Francisco, Department of Chemistry
29. Synthesis of an Array of
Glyopeptides for Characterization of Aptamer Selectivity toward
Post-Translational Modifications 2
Ryan Murray, Gregory R.
Stettler, Steven W. Suljak*
Santa
Clara University, Department of Chemistry and Biochemistry
30. Evaluation of Hand Held Data
Collection for General Chemistry Experiments. 2
Shirley Feng, Lawrence
Margerum*
University
of San Francisco, Department of Chemistry
31. Novel Biopanning Method for
Identification of Liver Cancer Protein-Ligand Interactions. 2
Laura Ramirez, Chun-Yi
Wu, Kit S. Lam*
University
of California, Davis, Department of Hematology and Oncology
32. Fluorescence Quenching of
Tris(2,2’-bipyridyl)ruthenium(II) in 0.5 M Aqueous Sulfuric Acid by Copper (II)
and Iron (III) at Room Temperature. 2
Deena Sadeli, Nina
Huynh, Silvio Rodriguez*
University
of the Pacific, Department of Chemistry
33. Preparation and Structural
Characterization of Peptoids with Biarsenical Dye Binding Motifs. 2
Myrna Mungal, Kanwal Palla, Lauren
Sartor, Amelia Fuller*
Santa
Clara University, Department of Chemistry and Biochemistry
34. Development of an Economically
Efficient Route to the Amino Acid Statine. 2
Michael Vu, James
Miranda*
California
State University, Sacramento, Department of Chemistry
35. Total Synthesis of Kavain. 2
Namphuong Nguyen, Stella
Plukchi, Claudia G. Lucero*
California
State University, Sacramento, Department of Chemistry
36. Aryl-Aminations Strategies
to Prepare Aza-Bridged Calixarenes. 2
Joshua Flynn, Chris
Stains, John Spence*
California
State University, Sacramento, Department of Chemistry
37. Characterizing the Surface
Chemistry Effects of Organically Modified Silica on Apomyoglobin. 2
Phillip Calabretta,
Mitchell Chancellor, Daryl Eggers*
San
Jose State University, Department of Chemistry
38. Intramolecular
Vinylcyclopropane Radical-Cyclization Fragmentation. 2
Andrea Bailey, Maddy
McCrea-Hendrick, James Miranda*
California
State University, Sacramento, Department of Chemistry
39. Progress Towards the
Synthesis of a Novel Boron Heterocycle. 2
Dung N. Nguyen, Frank E.
Cappuccio, Robin Baginski, Michael P. Groziak*
California
State University East Bay, Department of Chemistry and Biochemistry
40. Effects of Gel
Encapsulation on Corallina vancouverensis Acetone Powder (CVAP) Bromoperoxidase
Activity 2
William Wung, John H.
Kim, Ben Angunsri, Rei Hoang, Roy Okuda*
San
Jose State University, Department of Chemistry
41. Analysis of Chemical
Degradation on Solid Adsorbent Cartridges. 2
Jason S. Fell, Bradly M.
Baker*
California
State University, Sacramento, Department of Chemistry
42. Highly Conjugated
Arenediynes: Phenanthrenyl-Substituted Enediynes. 2
Calvin W. Ruger, Michael
L. Chang, John D. Spence*
California
State University, Sacramento, Department of Chemistry
43. Synthesis and
Characterization of Nb-doped Silicotitanate Materials. 2
Nicholas P. Genovia,
Jacqueline R. Houston*
California
State University, Sacramento, Department of Chemistry
44. Enhanced Photo-Bergman
Cyclization of 1-Ethynyl-2-Phenylethynylbenzene. 2
Nicholas P. Genovia,
John D. Spence*
California
State University, Sacramento, Department of Chemistry
45. β-thujaplicin
Production in Cupressus dupreziana Callus Cultures treated with
Methyl Jasmonate. 2
Kulvir Sarai, Thomas
Savage*
California
State University, Sacramento, Department of Chemistry
46. Using a Yeast Screening to
Identify SIRT Inhibitors from Marine-Derived Actinomycetes. 2
Van Pham, Jing Xiao,
Erika Tada, Taro Amagata*
San
Francisco State University, Department of Chemistry and Biochemistry
47. Exploration of Class I and
II HDAC Inhibitors from Marine-Derived Actinomycetes. 2
Erika Tada, Van Pham,
Jing Xiao, Taro Amagata*
San
Francisco State University, Department of Chemistry and Biochemistry
48. Isothermal Calorimetric Studies
of Chromium (III) with Various Ligands. 2
Nicole Trimble, Carmen
Works*
Sonoma
State University, Department of Chemistry
49. Analysis of the formation
of insoluble aggregates by the amyloid apolipoprotein variant L178H and WT.. 2
Mustafa Safi, Mai Thao,
Linda M. Roberts*
California
State University, Sacramento, Department of Chemistry
50. Investigations into
Trapping Carbocationic Intermediates in Ionic Liquids. 2
Sara Jaka, Beth Kochly*
Mills
College, Department of Chemistry
51. Fluorescent, solvatochromic
peptoid side chain: Synthesis and utility as a putative structural probe. 2
Paul Bruno, Marisa A.
Plescia, Amelia A. Fuller*
Santa
Clara University, Department of Chemistry and Biochemistry
52. Synthesis and Bergman
Cyclization of Butadiynyl Arenediynes. 2
Nicola Clayton, John D.
Spence*
California
State University, Sacramento, Department of Chemistry
53. Cloning the Human Vitamin D
Receptor into the pE-SUMOstar Expression Vector. 2
Lily Le, Aileen
Espinoza, Amanda Rodriguez, Charae Gilbert, Elaine D. Collins*
San
Jose State University, Department of Chemistry
54. Characterization of a
Chromium Binding Protein. 2
Jenna Bernard, Bernice
Wright, Carmen Works*
Sonoma
State University, Department of Chemistry
55. Bioinformatic
Identification of Nucleotide Sequences Possibly Encoding Enzymes in the
Biosynthetic Pathway of Domoic Acid 2
Mary Berard, Tom Savage*
California
State University, Sacramento, Department of Chemistry
56. Syntheses and
Photo-Reactivity of Methoxynaphthyl-Substituted Enediynes. 2
Trang Nguyen, John
Spence*
California
State University, Sacramento, Department of Chemistry
57. The amyloid apolipoprotein
variant L178H does not bind the beta-amyloid detecting dye ThT.. 2
Angela Monterrubio,
Linda Roberts*
California
State University, Sacramento, Department of Chemistry
58. Spectral Analysis of
Polylysine Structure in Organically-Modified Silica Glass Pores. 2
Gary Abel, Jr., Daryl K.
Eggers*
San
Jose State University, Department of Chemistry
59. Diastereoselective
acylation of 2-substituted cyclohexanols. 2
Jasper Visser, Andrey
Samoshin, Vyacheslav Samoshin*
University
of the Pacific, Department of Chemistry
60. Hofmeister Series Analysis of Tryptophan Using the Non
Interaction Model 2
Melinda D. Mendolla,
Daryl K. Eggers*
San
Jose State University, Department of Biochemistry
61. Bioinformatic determination
of the enzyme responsible for the double bond isomerization in the biosynthesis
of domoic acid in Pseudo-nitzschia multiseries. 2
Kelli Walker, Tom
Savage*
California
State University, Sacramento, Department of Chemistry
62. Bioinformatic
identification of nucleotide sequences encoding enzymes potentially catalyzing
the initial reaction in the biosynthesis of domoic acid in Pseudo-nitzchia multiseries. 2
Meghan Paul, Tom Savage*
California
State University, Sacramento, Department of Chemistry
63. UV-Vis Spectroscopic Studies
of Second-Coordination Sphere Interactions between Rutheniumamminepyridyl
Complexes and Dissolved Polyvinylpyrrolidone in Acetonitrile. 2
Vanessa Cristina Yu, Zhu
Li, Meng Shengnan, Jeff C. Curtis*
University
of San Francisco, Department of Chemistry
64. Intervalence
Charge-Transfer Transtions in Binuclear Ruthenim Ammine Complexes as Probes of
How Dissolved Salts Affect the Structure of Liquid Water. 2
Jerry W. Asuncion,
Anthony G. Fabrizio, Bryant J. Blair, Zhiji Han, Jeff C. Curtis*
University
of San Francisco, Department of Chemistry
65. Analysis of
Structure-Function Relationship in Taq DNA polymerase. 2
Maricela Salcedo,
Katherine Grutas, Lyly Doan, Matt Ono,
Patrick Batoon, Therese Nguyen, Joshua Espejo, Elaine Chau, Jerry Tsai*
University
of the Pacific, Department of Chemistry
66. β-Lactosyl Amine
Glycodendrimer Synthesis by Means of Amide Linkages. 2
Vincent Trapani, Doug
Glick, Katherine McReynolds*
California
State University, Sacramento, Department of Chemistry
67. Preparation of Carbon
Nanotube Based Device and Its Electrochemistry. 2
Khanh-Van Tu, Weiling
Hsieh, Daniel Straus, Roger H. Terrill*
San
Jose State University, Department of Chemistry
(Note: In each
abstract, the presenting author is underlined and the faculty mentor(s) is
denoted with an asterisk.)
a. University of San Francisco, Department of Chemistry
The hydroxyl (OH) radical
oxidation of 2-methyl-1-butanol (2M1B) is considered to be a good substitute
for petroleum fuels. The possible products are analyzed via multiplexed
chemical kinetic photoionization mass spectrometer, using the third generation
synchrotron light source from the ALS to photoionize the reaction species,
reactants, products, and intermediates. The products were identified by the
photoionization efficiency (PIE) curves. These curves are the plot of the ion
signal versus the photon energy from the ALS. Also, the PIE curves of different
molecules have different shapes due to their specific Franck-Condon factors.
The reactions are initiated by hydrogen abstraction from the OH radical,
followed by hydrogen loss (formation of a double bond), or carbon-carbon bond break
to form more stable products.
San Jose State
University, Department of Chemistry
Sialic acids are involved
in numerous important biological processes including cell adhesion. Cancer cells metastasize through
extravasation, the same mechanism macrophages use to exit the blood stream to
gain access to infected tissue.
Extravasation is a process requiring non-covalent interactions between
sialyl Lewis X (SLex) present on cell surfaces with E-/P-selectins
and integrins on the vascular endothelium to bring about rolling and firm cell
adhesion, respectively. Dafik et al., has demonstrated that cultured cells
incubated with fluorinated sialic acids exhibit decreased adhesion to
fibronectin, E-, and P-selectin. However, the molecular mechanism of this
reduced adhesion has not been established.
To determine whether the
observed decrease in adhesion is due to diminished binding of the fluorinated
SLex to the cognate lectin or to some other cellular compensatory
mechanism, we are interested in performing binding studies with chemically
homogeneous SLex analogues. To this end we are engaged
collaboratively in the synthesis of various fluorinated SLex
analogues. In this poster we report our
progress in the synthesis of fluorinated and non-fluorinated sialic acid
precursors that can be incorporated into the synthetic SLex
analogues. Once synthesized, the chemical
adhesion between these novel SLex analogues to E- and P-selectins
will be measured.
Qi Mo, R. David Britt*
University of California, Davis, Department of Chemistry
The effect of ammonia (NH3)
on the photosynthetic oxygen evolution has been examined by the pulse electron
paramagnetic resonance (EPR) technique of electron spin-echo envelope
modulation (ESEEM). O2-evolving Photosystem II (PSII) membranes
(concentration ≈ 2.5 mg chlorophyll/mL) were prepared by the method of
Berthold, Babcock, and Yocum. Both as-isolated and ammonia-treated samples were
studied by EPR after being trapped in the S2 state of the oxygen evolution
cycle. A change in the “multiline” Mn EPR signal was observed in the
ammonia-treated sample, which indicated that the ammonia was indeed binding to
the Mn ions in the PSII protein complex. This experimental result revealed that
there is a direct coordination of an ammonia-derived ligand to the catalytic Mn
complex during the S1 to S2 transition of the oxygen evolution S-state cycle.
Our next goal is to determine if interactions of other ligands with the Mn
complex—namely, putative histidine ligands—are also altered upon treatment with
ammonia. These interactions will be measured by CW(Continuous Wave) EPR
spectroscopy.
San
Jose State University, Department of Chemistry
Chiral recognition is of
utmost importance in the synthesis and characterization of novel
pharmaceuticals and small molecules where a difference in chirality can
determine the desired drug action and sometimes cause a drug to become
effective or ineffective. Circularly Polarized Luminescence spectroscopy (CPL)
has been shown to have the ability to characterize and potentially quantify the
enantiomeric make-up of product mixtures. Luminescent chiral lanthanide(III)
complexes have shown promise as potential molecular probes to use with CPL. In
this study, a derivative of dipicolinic acid is being characterized in terms of
pKas, luminesence properties, and complexation with Tb(III). Once the current complexation model is fully
verified, the photophysical properties of various forms of the complex will be
determined. The pKas of MEBADPA and the stability constants of each complex
need to be determined by titration. Each
system is aromatic and/or emissive, so spectrophotometric titrations were used
as well as potentiometric after issues were encountered with the complexation
data. An automatic pumping system was
used to perform titrations, and the data were refined by the HYPERQUAD2006
package. In this study, a selected lanthanide(III)-based system is being
developed and characterized in order to expand the tool set for CPL-based
systems used as probes for biomolecules. The MEBADPA ligand was complexed with
terbium(III) and the stability constants are found, which will allow the
complete characterization of the ligand.
University of California, Davis, Department of Chemistry
Liposomes are becoming
increasingly prevalent as an important part of drug delivery systems in modern
medicine, however the need for a better understanding of the physical
characteristics are needed. In this
study we present our results on the stability and adsorption of liposomes
formulated from dipalmitoylphosphatidylcholine (DPPC) via attenuated total
reflectance Fourier transform infrared (ATR-FTIR) spectroscopy. The phase transition temperature of pure DPPC
liposomes, DPPC liposomes containing cholesterol, and DPPC liposomes containing
cholesterol and polyethylene glycol (PEG) is determined using a temperature
dependant study (25°C to 50°C) and been found to occur abruptly at
approximately 41°C for pure DPPC liposomes,
gradual changes between 35°C to 43°C for DPPC liposomes containing
cholesterol, as well as the PEGylated liposomes. The rate of adsorption of these liposomes
onto various hydrophilic and hydrophobic surfaces was also studied and is
presented here. Knowing the stability
and adsorption characteristics of these liposomes allow for a better
understanding of their use in drug delivery systems.
University of California, Davis, Department of Biochemistry
Cardiomyopathy is a
disease of heart muscle that is diagnosed in 1 out of every 500 individuals. In
particular, Hypertrophic Cardiomyopathy (HCM) is the leading cause of sudden
cardiac death in athletes and is characterized by abnormally thick left ventricle
of the heart. Contrarily, Restrictive Cardiomyopathy (RCM) is a rare stiffening
of the muscular walls of the heart. These conditions have been linked to
genetic mutations in several proteins including Human Cardiac Troponin (Tn), a
sarcomeric protein complex that regulates heart muscle contraction. The
synchronized contraction of cardiac myocytes is initiated by the release of Ca2+
from sarcoplasmic reticulum and is followed by structural changes of Tn that
reveal the actin-myosin binding site for force development. The latter is
closely related to the Inhibitory subunit of Tn (HcTnI), which, in the absence
of Ca2+, blocks myosin binding sites on actin. Research shows that
point mutations R145W and R145G in the inhibitory region of HcTnI are linked to
RCM and HCM, respectively; both mutations impair the proper response of HcTnI
to Ca2+ resulting in a significant increase in Ca2+
sensitivity. Phosphorylation sites on TnI have shown to decrease Ca2+
sensitivity. We hypothesize that phosphorylating Serine residue number 151
located in the inhibitory region of HcTnI to Alanine or Glutamic Acid (S151A/E)
may counteract R145G/W mutations by modulating Ca2+ sensitivity. To
test the functional outcome of S151A/E phosphorylation, we utilized several
methods including site-directed mutagenesis, DNA purification, protein
purification, reconstitution of Tn complexes, ATPase assays, and Calpain
digestions.
San
Jose State University, Department of Chemistry
Cytochromes P450 are a
superfamily of heme-thiolate monooxygenases, found in almost all living
organisms, that play an important role in the biosynthesis and biodegradation
of endogenous compounds. The classical P450 reaction is the introduction of an
oxygen atom, derived from molecular oxygen, into a substrate unactivated carbon
center. The mechanism involves the reductive scission of the O-O bond at the
iron center, leading to the formation of a highly oxidative heme radical ferryl
species, Compound I.
The long-term goal of
this research is to develop hybrid P450 heme domain enzymes that will utilize
light and water to perform selective hydroxylation of C-H bonds in various
substrates under inert atmosphere. A photosensitizer Ru(II) diimine compound
covalently attached to the heme domain enables the photo-oxidation of the
resting state, heme Fe(III)-aqua complex, to generate high-valent Fe(IV)-oxo
species. Substrate hydroxylation will occur through a novel oxidative pathway
rather than through the reductive pathway involving molecular dioxygen used by
the enzyme and the current studies. This new approach will provide enhanced
activity of the heme domain as a result of 1) a better control of the electron
flow and 2) a lack of rapid enzyme deactivation due to the absence of reactive
oxygen species.
San
Jose State University, Department of Chemistry
Cytochrome P450s are
heme-thiolate enzymes that play an important role in the biosynthesis and
biodegradation of endogenous compounds. The classical P450 reaction is the
regio and stereo selective introduction of an oxygen atom, derived from
molecular oxygen, into a substrate unactivated carbon center. Over the years,
great advances have been made in mimicking P450 reactivity by utilizing metal
(Fe, Mn, Ru) porphyrins and single oxygen atom donors (iodosylbenzene,
peracids, hydrogen and alkyl peroxides). However, these models still lack 1)
substrate recognition properties leading to regioselectivity of oxidation and
2) control of electron-proton transfer steps when oxygen is used as
oxidant.
Molecular Imprinted
Polymers (MIPs) are highly porous materials with template-shaped cavities. MIPs
have had great success in the field of chemical sensing but only scarce examples
of catalytic polymers are available. A MIP, which encompasses a small molecule
catalyst and a templated cavity for a specific substrate would utilize the
advantages of both enzymes and small molecule models with the incorporation of
the active site within a defined geometry capable of performing reactions and a
greater tolerance of pH, temperature, and organic solvents. We will present our
recent findings in incorporating and characterizing a porphyrin moiety into a
polymer matrix.
California State
University, Sacramento, Department of Chemistry
The classic Ritter
reaction involves the interaction of a nitrile with cation generated by either
an alcohol or an alkene under acidic conditions to produce an N-substituted
amide. Modified Ritter reactions have
been developed which do not rely on the traditional cation sources. In this study, a modified Ritter reaction was
undertaken in which various nitriles were allowed to react with an ester in
order to generate the corresponding t-butyl amide products utilizing a novel,
microwave-driven synthesis. Substituent effects on the nitrile were examined in
order to understand the reaction outcome and assess the versatility of this
reaction.
Sonoma State
University, Department of Chemistry
This work describes the
use of atom transfer radical polymerization (ATRP) in the synthesis of Fe3O4/polystyrene
(PS) core/shell nanoparticles. In this
approach, oleic acid stabilized Fe3O4 nanoparticles were
ligand exchanged with 2-bromo-2-methylpropionic acid, the initiator for
ATRP. These Fe3O4
nanoparticles are soluble in styrene and were used as macroinitiators for
ATRP. Well-defined Fe3O4/polystyrene
core/shell nanoparticles were obtained.
The synthesized core/shell nanoparticles were characterized by scanning
electron microscopy (SEM).
San
Francisco State University, Department of Chemistry & Biochemistry
Current PVC plasticizers are
predominantly phthalate esters which are known endocrine disruptors. Although these plasticizers have been banned
in children’s toys, they are surprisingly still found in medical devices such
as dialysis tubing and blood bags because general, suitable replacements have
not yet been found. A promising newer
strategy involves non-migrating plasticizers which cannot leave the polymer
matrix. Current approaches involve
polyesters which are prone to hydrolysis, which degrades physical properties
and releases potentially hazardous small molecules. To avoid these pitfalls, we are exploring
PE-PMMA co-polymers with polar ester groups and hyper-branched backbones as
non-migrating plasticizers for PVC.
These are best synthesized in controlled fashion with Pd Monomethyl
Diimines which are unique in their ability to insert both ethylene (non polar)
and polar monomers such as acrylates while introducing branches into the
poly(olefin) which impart flexibility and processibility. Unfortunately, the current literature
method to prepare these valuable catalysts requires tetramethyl tin, a volatile
and highly toxic reagent whose byproducts are difficult to completely
remove. The work presented here
demonstrates a new, practical, and relatively ‘green’ synthetic route to these
molecules which avoids these hazards.
The merits and challenges of several alternative approaches will be
discussed.
California State
University, Chico, Department of Chemistry
The Zhang group is
working towards the total synthesis of neolignans isolated from Rodgersia
podophylla A. Gray (Saxifragaceae). While model compounds have been
successfully synthesized using an inverse electron demand hetero-Diels-Alder
reaction, the regioselectivity of the method has been poor.
We have characterized
compounds that have been synthesized with numerous methods; commonly used are
nuclear magnetic resonance, both 1H and 13C, X-ray
diffraction, high pressure liquid chromatography with UV-Vis detection, and gas
chromatography/mass spectrometry.
California State
University, Sacramento, Department of Chemistry
The catalytic reaction of
the metalloenzyme peptide deformylase, which involves the cleavage by hydroxide
of the formyl group at the N-terminus of nascent proteins during peptide
synthesis, has been suggested to be vital for eubacterial cell growth. As a
potential antibacterial target it is imperative to study the catalytic process
in detail. A computational investigation of the catalytic reaction using
density functional theory was carried out using a biomimetic model consisting
of a previously proposed heteroscorpionate ligand complexed to an Fe(II)
center. The investigation was focused on altering the ligand through
substitution of various electron-donating and electron-withdrawing groups and
observing the immediate effects on the reaction thermodynamics and kinetics. It
was found that the reaction free energy increased such that the product was
more unfavorable as the added substituents shifted from electron-donating to
electron-withdrawing. An electronic investigation suggests that the
hydroxide-coordinated complex becomes further stabilized with
electron-withdrawing substituents as the hydroxide ligand is pulled closer to
the Fe(II) center. After determining the transition states, it was found that
the activation energy of the nucleophilic attack step experiences a quadratic
correlation with a rise in energy as the substituents’ electronic donating or
withdrawing abilities increase.

California State
University, Sacramento, Department of Chemistry
Inorganic ion-exchange
materials have been used for decades as water softening agents, sorbents, and
for the selective removal of radioactive isotopes. Titanosilicate is a
structural analogue of the mineral pharmacosiderite, a non-aluminosilicate
molecular sieve that is well suited as an ion-exchanger. Titanosilicate
pharmacosiderites (TSP’s) have a unique framework caused by the alternating
titanium octahedra and silicate tetrahedra that create a three-dimensional
tunnel system filled with exchangeable ions. This study presents the
hydrothermal synthesis and solid-state characterization (XRD, FTIR, solid-state
NMR) of various TSP’s in the cesium phase, doped with 4-coordinate or
6-coordinate germanium. The substitution of germanium into these sites has been
shown to cause distortions within the crystal structure that may effect the
tunnel size and by default, ion-exchange selectivity. Future studies will focus
on the ion exchange properties of these novel ion-exchange solids.
Xanthene dyes tend to
aggregate even in dilute aqueous solutions causing dimer formation, or higher
order aggregates, which are strongly affected by structure, concentration,
ionic strength, temperature, and the presence of other organic molecules.
Rhodamine 6G was studied as a function of temperature in the range of 10 - 75°C
in the absence and presence of ~2.0 M aqueous sodium chloride. The presence of electrolytes clearly induces
dimer formation. The DATAN (DATa
ANalysis) 3.1 software was used to determine the relative amounts of monomer
and dimer present in solution, and the equilibrium constant for dimerization,
in the presence of electrolytes, is 8400.
The Gibbs free energy of dimer formation is -22.4 kJ/mol and a van’t
Hoff plot gives a standard enthalpy of dimerization of -39.2 kJ/mol. Then the
standard entropy of dimer formation is calculated to be -56.5 J/(mol*K).
University of San Francisco, Department of Chemistry and Environmental
Science
A computational study (ab
initio and DFT) of numerous dehydro[12]annulene isomers reveals the most stable
isomer to be 1 (CTCTC
configuration). Two additional configurations of dehydro[12]annulene were
located that were only 4 kcal/mol higher in energy, 2 and 3
(CCSD(T)/cc-pVDZ//BHHLYP/6-31G*). Computed energies along with computed 1H
NMR values and coupling constants are at odds with reports that different
isomers of dehydo-[12]annulene have been synthesized.

Calix[4]arene and its
derivatives are an interesting class of molecules that can provide a
hydrophobic "bowl" to interact with small molecules or metal ions in
supramolecular host-guest chemistry. However, their potential as an artificial
receptor for nucleic acid components (nucleobases, nucleosides, and nucleotides)
has not yet been fully examined. We hereby report the synthesis and
characterization of nucleobase-calix[4]arene conjugates using "click"
chemistry. These molecules maintain a cone conformation as determined by 13C
NMR. Their complexation with nucleobases in the gas phase using ESI mass
spectrometry will also be discussed.
Sonoma State
University, Department of Chemistry
Pharmacological
administration of nitroxyl (HNO) has been shown to affect a number of
physiological functions. Of its many
functions, one of high importance is its ability to be used as a treatment of
various cardiovascular disorders. Though
very useful once within a physiological setting, HNO by itself is unstable and
therefore must be administered using HNO-donor compounds. Some HNO-donating compounds include: Angeli’s
salt, Piloty’s acid and, the one of increasing interest, acyloxy nitroso
compounds. In our research, we have
focused our efforts on creating a number of acyloxy nitroso compounds for
comparison of release of HNO with other various donors. We have seen that by altering various
molecular components of the acyloxy nitroso compound, we can affect the release
of HNO from these donors. Furthermore,
use of polar or charged oximes in the production of the acyloxy nitroso
compound is also expected to produce a water-soluble species, which in turn,
can be directly applied in a physiological setting.
Santa
Clara University, Department of Chemistry and Biochemistry
Inverse mini-emulsions
containing water-soluble acrylamide monomers were established. These free
radical polymerization in these emulsions result in high molecular weight
polymer particles of controlled size and composition. We have explored range of
cross-linking densities and composition of poly(acrylamide-co-N-,N- dimethyl
acrylamide) in the colloids. We study the swelling behavior of the particles as
a function of pH and solvent conditions using dynamic light scattering (DLS)
and zero-shear viscometry. We study these nano-gels as potential dense sieving
matrices for stationary phase capillary electrophoresis. Result from the
synthesis, purification and size analysis will be presented.
Santa
Clara University, Department of Chemistry and Biochemistry
This project has the
overall goal of forming a biocompatible polymer shell around a nanometer-sized
water droplet. These polymer shells are of interest as potential bio-molecule
containing vesicles. In this project we have focused on: 1) Formulation of an
inverse meta-stable emulsions that have sufficient stability to serve as
capsule templates. 2) Construction of a thin polymer shell around the micelle
droplets to freeze the capsule structure and stabilize them in a greater range
of environments. A specific choice of polymer shell was made to form a
semi-porous nano-gel layer rather than a dense plastic layer. 3) Isolation and
purification of the capsules and dispersion in water. These particles may
function as nano reactors, which may be of great interest to the medical field
nano-drugs.
California State
University, Chico, Department of Chemistry
Recently a series of nine
structurally related acetylenic carboxylic acids, gallicynoic acids, have been
isolated from basiomycete Coriolopsis gallica.
Some of these acetylenic metabolites exhibit diverse bioactivities,
including cytotoxic, antimicrobial, enzyme-inhibitory, and anti-HIV
activities. We have modified a previous
synthetic scheme in our attempts to produce these acetylenic acids in a racemic
fashion. Our previous scheme featured ruthenium catalytic conjugate addition of
terminal acetylenes and Sonogashira cross-coupling of terminal acetylenes to
unactivated alkyl bromides and iodides that failed to produce the desired
hetero-coupled products. Our revamped synthetic scheme giving our desired
racemic products will be presented. We are currently devising enantioselective
an acetylide/aldehyde process that will lead to non- racemic gallicynoic acids
with the desired stereochemistry.
Sonoma State
University, Department of Chemistry
Photochemical studies of
an iron-only hydrogenase model compound, Fe2(SCH2CH2CH2S)(CO)6
(the model), was investigated. Photochemical and thermal reactivity were
studied in acetonitrile (MeCN) for the model under air, nitrogen and CO. Results from both studies are consistent with
the loss of CO from the model upon photolysis and the generation of a solvento
species. Studies presented here are concerned with varying concentrations of
CO, monitoring of the kinetics and measuring the observed rate constant for the
reaction of the photo-product with CO in MeCN as well as CNCH3
(tetrahydrofuran) at room temperature. These studies yielded second order rate
constants of 2.0E-5 mM-1 s-1 and 9.0E-4
mM-1 s-1 respectively for the two solvents. Results
suggest the formation of a solvento species that reacts with CO to reform the
parent complex. Kinetics data at different temperatures will also be presented.

California State
University, Chico, Department of Chemistry and
Biochemistry
Monobrominated cyclohexanones,
like their unsubstituted parent compounds, readily undergo chair-chair
interconversions. An example is
2-bromo-3,3,5,5-tetramethylcyclohexanone (1) shown below. The relative proportion of the axial versus
equatorial conformations is strongly dependent on the polarity of the
solvent. In previous work the 1H
NMR, IR and UV-Vis spectroscopic techniques have been applied to quantify the
dynamical interconversion, but the validity of this approach is still under
investigation. We will present detailed computational results that parallel the
experimental 1H NMR results on (1) and on model compounds to further
our understanding of this system.

California State
University, Sacramento, Department of Chemistry
Plants are the major
source of volatile organic compounds (VOC) to the atmosphere and play a major
role in the chemistry of the lower atmosphere.
Of major interest is the contribution that biogenic VOCs make to natural
background organic aerosol when they are oxidized in the atmosphere. Some VOC compounds are ‘sticky’ and have a
short lifetime, some are polar and others are not, all of which make VOCs
difficult to sample and quantify. Solid
phase microextraction (SPME) fibers avoid much of the sample handling obstacles
encountered with other gas sampling techniques.
This study specifically focused on six different SPME fibers, each
containing different coatings or film thickness, to determine which standard
VOCs were observed, at what time they reached equilibrium, and what the
partition coefficient was. The various
fibers (100 and 7 µm PDMS, 65 µm PDMS/DVB, 85 µm PA, 85 µm CAR/PDMS, 50/30 µm
DVB/CAR/PDMS, 60 µm PEG) were tested against various sesquiterpene,
monoterpene, and wound compound standards as well as isoprene, and
2-methyl-3-buten-2-ol. Analysis was accomplished by gas chromatography mass
spectrometry for identification and flame ionization detection for
quantification of the VOCs.
California State
University, Sacramento, Department of Chemistry
The mediated
electroreductive cyclization (ERC) reaction allows the substitution of an
electrocatalyst as the stoichiometric source of electrons rather than the
cathode, with the advantage of enabling chemoselectivity through the use of a
more positive potential than necessary in the direct substrate reduction. Metal
salens can play the role of electrocatalysts for mediated ERC reactions,
provided that they operate within a specific potential window where electron
transfer can take place. The mechanism of these reactions is unknown, but it
has been postulated that inner sphere electron transfer is a likely pathway.
Nickel salen can be used as an electrocatalyst for the ERC reaction. A study of cyclic voltammetric properties of
reduced Nickel salen in the presence of an α,β-unsaturated carbonyl
compound was employed to analyze the mechanism of Nickel salen’s catalytic
behavior in ERC reactions. Bulk electrolysis was also employed using methyl cinnamate
in an attempt to isolate a modified electrocatalyst and verify the inner sphere
electron transfer mechanistic pathway.
California State
University, Sacramento, Department of Chemistry
The goal of this project
is to determine the rate at which water ligands in the metal complex [M3(μ3-O)2(μ-O2CCH3)6(OH2)3]+2
(M = Mo, W) are substituted by CD3OD using 1H NMR
spectroscopy. The tetravalent metal ion
was varied to determine the effect on ligand substitution. Rates of water substitution were determined
by plotting the 1H NMR signal area from the methyl group on the
carboxylate bridge as a function of time.
Preliminary data indicates that water substitution occurs faster for
molybdenum complexes. Future work will
focus on varying the functional group on the carboxylate bridge to investigate
its effect on ligand substitution.
California State
University, Sacramento, Department of Chemistry
1,2,4-Triazoles represent
an important structural element in a variety of useful molecules with
applications varying from industrial (corrosion inhibitors) to medicinal
(anti-bacterial, anti-inflammatory,
anti-microbials). Triazoles are particularly
interesting groups in pharmaceutical applications since they are more likely to
be water soluble than other aromatic systems and are stable under biological
conditions. With multiple nucleophilic
sites, the 3-amino-1,2,4-triazole derivatives make for excellent candidates for
tandem cyclization reactions. The fused
heterocycles which are then produced can be studied as analogs to natural
occurring bi-cyclic ring systems such as purines. In this study, the reactivity of
aminotriazoles towards tandem cyclization reactions with a variety of
difunctional electrophiles is explored.
University of San Francisco, Department of Chemistry
The purpose of this study
was to computationally locate a [10]annulene minimum with a figure either
(two-twist) topology. Two benzene rings
were fused to the two twist core of [10]annulene to form four different isomers
(1-4). At the
CCSD(T)/6-31G*//BHHLYP/6-31G* benzo-fused (1) was lowest in energy, with
species (2) only .7 kcal/mol higher in energy.
Bond length analysis revealed that all structures displayed bond
alternation (∆r= 0.15-0.16Ĺ). The
barriers for non planar pi-bond shifting for these species were all lower in
energy than the barrier for the unsubstituted system. When a two-carbon bridge was also included
(5), the barrier of the [10]annulene core of (5) was reduced to 3 kcal/mol.

Santa
Clara University, Department of Chemistry and Biochemistry
Our research is focused
on the development and characterization of highly selective aptamers that can
discriminate between peptides with variant post-translational modifications. As
a method of modeling these modifications, we have begun to synthesize, using
solid phase peptide synthesis, a panel of glycopeptides that vary in both the
identity and location of their sugar moities. Our target peptides mimic a 32mer
fragment of vascular endothelial growth factor (VEGF), which includes the
asparagine residue that is glycosylated on the native protein. To enhance
efficiency, we have replaced this asparagine residue with an unnatural amino
acid that contains an N-alkylaminooxy side chain, which has been reported to be
an effective nucleophile for chemoselective glycosylation. Herein we report our
progress on constructing the array of VEGF glycopeptide mimics.
University of San Francisco, Department of Chemistry
The Vernier LabQuest is a
stand-alone hand-held device with built-in data acquisition and analysis
software. We investigated standard USF
General Chemistry experiments using both manual data acquisition and with
LabQuest to examine the efficiency of the device. Two experiments, "Kinetics of Dye
Bleaching" and "Microscale Acid-Base Titration" were carried out
using both methods. Our results indicate
that by using LabQuest, accurate data can be obtained with time and chemical
waste greatly reduced. Students should
be able to obtain good data more quickly, generate less chemical waste and have
time to repeat poor results that was not previously obtainable.
University of California, Davis, Department of Hematology and
Oncology
The screening of one-bead-one-compound
(OBOC) combinatorial chemistry libraries against a single target protein and
the screening of phage display libraries against a single ligand have both been
well developed. Recently, a novel multiplex screening method has been developed
to screen an OBOC small molecule library against a phage display cDNA library
to discover protein-small molecule binding pairs efficiently. The structures of
the small molecules have been decoded and each identified compound has been
re-synthesized on tentagel beads in large quantities for the convenience of
biopanning. We panned the T7 phage-display liver tumor cDNA library onto the
small molecules immobilized on the beads, washed out the unbound phages, and
eluted the bound phages by infecting E. coli BLT-5403. The bound phage
populations were amplified for another round of panning. Four rounds of phage panning and
amplification were performed for each ligand to enrich the phage populations
that carry proteins that bind specifically to the ligand. Single plaques from T7-infected E. coli were
isolated, representing phage corresponding to each ligand. Polymerase chain reaction (PCR) amplification
for DNA sequencing of phage plaques and protein BLAST was performed to identify
target proteins. The protein-small
molecule binding pair database has been established, and it may be useful for
discovering novel protein inhibitors against cancers, developing novel
diagnostic tools, or further proteomic studies.
The fluorescence
quenching of Tris(2,2’-bipyridyl)ruthenium(II) cation by copper (II) and iron
(III) has been studied at room temperature in aqueous 0.5 M sulfuric acid.
Under these conditions, the Stern-Volmer constants Ksv are determined to
be 25.8 1/M for copper (II) and 734 1/M
for iron (III). These are diffusion controlled processes, and the quenching
rate constants and the self-exchange rate constants for the cations are
analyzed in the context of Marcus theory.
Santa
Clara University, Department of Chemistry and Biochemistry
Biarsenical dyes (e.g.,
FlAsH, ReAsH) are nonfluorescent until they bind covalently to tetra-thiol
motifs in proteins. Although these dyes have been shown to be useful probes of
protein secondary or tertiary structures, they have not been used to study
abiological molecules. As part of a long-term effort to recapitulate complex
protein structure and function wtih abiological molecules, we hypothesize that
biarsenical dye fluorescence will correlate with secondary or tertiary
structural features of peptoids (N-alkyl glycine oligomers). Peptoids
containing potential biarsenical dye binding motifs have been prepared and
structurally characterized. These include peptoids with varied helical
propensities and peptoid amphipathic helices proposed to form a dye-binding
motif upon self-association.
California State
University, Sacramento, Department of Chemistry
The objective of this
project is to develop an efficient, large scale synthetic route of the amino
acid statine. Previous syntheses of
statine suffer from lengthy routes and low diastereoselectivities in key
steps. Additionally, these routes
usually employed time consuming column chromatography. All of these factors contribute to the fact
that the current technology is not amenable to a large scale (kilogram)
synthesis of statine. This project seeks
to develop a highly stereoselective and efficient synthetic route in which
chromatography can be avoided completely.
Our collaboration with American Peptide Company in Sunnyvale, CA
seeks to develop a cost efficient synthesis. Our overall objective is to develop a
synthetic route to that can lead to 1 kilogram of statine at a cost of $30/gram
to produce.
Our approach towards the synthesis of the
unusual amino acid statine employs a combination of two earlier independent
strategies by Li and Hoffman. Our
strategy seeks to use a MgI2 diethyl etherate-catalyzed
Mukaiyama-type aldol reaction developed by Li and the use of a N,N dibenzyl
protecting group (Hoffman) in the key coupling step. We are hopeful that these combined factors
will serve to dramatically increase the observed selectivity in creating the 4S
stereocenter in statine.
California State
University, Sacramento, Department of Chemistry
The enantioselective
conjugate allylation of α,β-unsaturated aldehydes for the synthesis
of biologically active natural products will be investigated. The addition of
nucleophiles to α,β-unsaturated aldehydes prefers to proceed in a 1,2-fashion
rather than in a 1,4-fashion. Activation
of the α,β-unsaturated aldehydes with chiral imidizolidinones,
however, has proven to be successful in directing the addition in a
1,4-fashion. It is our goal to introduce the first enantioselective conjugate
allylation of α,β-unsaturated aldehydes using substituted allyl
indium reagents. This asymmetric approach will allow access to the natural
product kavain, the principle constituent of the kava plant, reported to
exhibit great promise in the treatment of ovarian cancer and leukemia.

California State
University, Sacramento, Department of Chemistry
Heterofunctionalized calixarenes
are a rapidly growing field in supramolecular chemistry. Heteroatoms may be introduced by replacement
of phenol building blocks with heteroaromatic rings or through substitution of
bridging methylene groups with heteroatoms.
As part of a program to selectively replace methylene bridges with free
amino groups, we recently described the synthesis of diazacalix[8]arene and
triazacalix[12]arene methyl ethers via intramolecular Buchwald-Hartwig aryl
amination of an open chain bromo-amino tetramer. In an effort to prepare smaller azacalixarene
derivatives, we are currently examining intramolecular aryl aminations
strategies of the corresponding bromo-amino dimer which can afford
diazacalix[4]arene and triazacalix[6]arene as well as higher homologs. Following our previous work, the requisite
amino-bromo dimer can readily be prepared in four steps from
4-tert-butyl-2-hydroxymethyl-6-nitrophenol.
Our preparation of this key intermediate and our ongoing studies towards
its cyclization will be presented.
San
Jose State University, Department of Chemistry
Encapsulation of a
protein in silica gel is a useful technique for preparing a protein to be
analyzed using spectroscopic methods, specifically circular dichroism which
reveals the secondary structure of a protein. The silica gives a transparent
medium for immobilization of the protein thus preventing aggregation under
denaturing conditions. Also, the glass mimics macromolecular crowding, which
occurs in vivo, by trapping proteins in the interconnected pores of the glass
matrix which prevents the protein from diffusing out but allows buffers to be
switched after a short equilibration period. The standard silica matrix
denatures our marginally stable reporter protein, apomyoglobin (apoMb).
However, on incorporation of organic modifiers, the glass becomes more
“biocompatible” allowing the protein to regain some of its native helices. By
varying the type (charged, polar uncharged, or nonpolar) and amount of the
modifiers, a large range of effects can be seen on the structure of the
encapsulated protein. Currently, focus is on low percentages (2.5-5 %) of each modifier
to better characterize the effect of each reagent on apoMb. We hypothesize that
the surface chemistry of the modified glass alters the average free energy of
confined water, thereby influencing the secondary structure of the protein.
California State
University, Sacramento, Department of Chemistry
Carbocyclic rings are
common in biologically-active natural products.
Paclitaxel, isolated from Taxus Brevifolia, is a powerful anticancer
agent which contains an eight membered carbocyclic ring. Caryophyllenes, containing nine membered
carbocyclic rings, are antileishmanials.
Germacranes are a class of compounds that contain a ten membered
carbocyclic ring. Germacranes can be
found throughout nature; in plants such as caraway, liverworts; various
arthropods; and fungi.
Entropic factors
complicate the synthesis of these medium sized rings. In our approach, cyclization of a radical
upon an appended vinylcyclopropane is followed immediately by a facile ring
opening of the cyclopropylcarbinyl radical to give the eight, nine, or ten
membered ring. Development of an
appropriate test substrate is key to testing our mechanistic hypothesis. Additional investigation focuses on the addition of a cycloenolate to the
natural product ethyl chrysanthemate.
Our methodology can be applied to natural products containing medium
sized carbon rings such as the anti-cancer compound paclitaxel.
California State
University East
Bay, Department of Chemistry and Biochemistry
We report our progress in
preparing a novel compound, 2-(hydroxycarbamoyl)phenylboronic acid. There is
current interest in boron-based molecules as potential new molecular
therapeutics. Efforts in our lab are currently underway to synthesize and study
this target’s structure in the solid state and in various solutions. Our goal
is to investigate how a hydroxamic acid will interact with an ortho boronic
acid. The multi-step synthetic route towards this new boron heterocycle is
described, and the first four of the six steps have been accomplished. In
addition, we have predicted a collection of potential cyclic structures which
our target might form. We will investigate the final structure of our target by
high field NMR and other analytic techniques.

San
Jose State University, Department of Chemistry
Gel encapsulation
provides a convenient and effective means of enzyme reuse over the course of
many reactions. This technique is
especially useful given the limited resource of many biologically useful
enzymes. To this end, we investigated
the effects of encapsulating Corallina vancouverensis acetone powder (CVAP),
which contains a bromoperoxidase, in sodium alginate and tetramethyl
orthosilicate (TMOS) sol-gels of different shapes and sizes. Enzyme activity was tested using a
colormetric phenol red assay.
Preliminary results indicate varying degrees of enzyme activity, which
in turn suggest that both enzyme distribution within a gel and total available
gel surface area affect reaction rates of encapsulated enzymes.
California State
University, Sacramento, Department of Chemistry
The use of solid
adsorbent cartridges is a common method for sampling analytes that are in the
gas phase. Sesquiterpenes (SQT) are
biogenic volatile organic compounds emitted by trees into the lower
atmosphere. SQTs react quickly in the
atmosphere which makes analysis difficult.
Using solid adsorbent cartridges for SQT analysis is ideal for both in
the laboratory and in the field because they trap SQTs before they react. However samples taken in the field may not be
analyzed for great lengths of time, which can cause sample degradation. In this study solid adsorbent cartridges containing
Tenax TA and Carbopack B were stored with a standard of 10 SQTs for 8 weeks to
analyze any sample degradation. 20 cartridges were stored and every 2 weeks 5
cartridges were analyzed on a Gas Chromatograph paired with Flame Ionization
Detection. The results show that there
appears to be no degradation of the SQTs sampled onto these solid adsorbent
cartridges when compared to a known SQT standard.
California State
University, Sacramento, Department of Chemistry
Over the past few years
our group has been interested in the syntheses of highly conjugated enediynes
to examine reactivity with ultraviolet and visible light. Towards this end, we
have prepared a number of arenediynes possessing aryl substituents on the
enediyne alkyne. In addition to increasing conjugation, incorporation of
bulkier alkyne substituents also increases steric hindrance towards Bergman and
the related C1-C5 cyclization. We are currently examining
the photoreactivity of arenediynes possessing one or two phenanthren-9-yl
substituents. Addition of two phenanthrenyl groups prevents enediyne
cyclization at 300 nm, while excitation of the phenanthrenyl chromophore at 350
nm leads to a novel bis 2+2 photo-dimerization. The reactivity of this system,
along with the synthesis and photoreactivity of the less hindered
mono-phenanthrenyl derivative, will be presented.
California State
University, Sacramento, Department of Chemistry
Recent studies of
inorganic ion-exchange solids such as crystalline silicotitanate(CST) materials
have shown them to be highly selective for the removal of radioactive 137Cs
in the presence of large amounts of brine. These studies have also shown that
doping the structure with Nb metal ions increases the unit cell of the crystal
lattice and as a result increases the solid's ion exchange affinity for large
alkali metal ions. Undoped and doped crystalline silicotitanate materials with
the formula HNa3Ti4Si2O14·4H2O
and HNa2Ti3NbSi2O14·4H2O,
were synthesized hydrothermally and analyzed using solid-state techniques (XRD,
FTIR, solid-state NMR).
California State
University, Sacramento, Department of Chemistry
The Bergman cyclization
of enediynes to produce 1,4-benzenoid diradical intermediates has been
attributed to the potent ability of natural products like calicheamicin to
cleave DNA. Photochemical Bergman
cyclization of 1,2-bis(phenylethynyl)benzene derivatives is a common strategy
employed to selectively activate enediynes towards cyclization by exposure to
light. The overall yield for cyclization
of the parent substrate, however, is very low as it suffers from significant
polymerization due to long irradiation times.
In an effort to improve cyclization yields of simple acyclic enediynes
we have re-examined the photochemical reactivity of
1-ethynyl-2-phenylethynylbenzene, which we recently reported as the first
example of a terminal arenediyne which undergoes photo-Bergman
cyclization. In addition to affording C1-C6
cyclization products in higher yields compared to
1,2-bis(phenylethynyl)benzene, 1-ethynyl-2-phenylethynylbenzene requires
significantly less exposure time and forms fewer byproducts. Our optimization studies for the
photocyclization of this mono-substituted arenediyne compared to
1,2-bis(phenylethynyl)benzene will be presented.
California State
University, Sacramento, Department of Chemistry
β-thujaplicin is an
antimicrobial tropolone synthesized naturally by Cypress trees. Understanding β-thujaplicin biosynthesis
is required to enable genetic engineering of disease resistance into
agronomically important plant species. Studies to elucidate the
β-thujaplicin biosynthetic pathway require a tissue culture system that
produces significant levels of β-thujaplicin. Methyl jasmonate has been
used to elicit higher levels of plant natural product biosynthesis in other
plant tissue culture systems. Here we evaluated the ability of methyl jasmonate
to elevate β-thujaplicin production in cultures of Cupressus dupreziana.
Production of β-thujaplicin was observed in both treated and untreated
cultures; however no significant increase in production of β-thujaplicin
was observed in methyl jasmonate treated cultures. These cultures may be
unresponsive to methyl jasmonate, or higher levels of methyl jasmonate may be
required to stimulate β-thujaplicin production.
San
Francisco State University, Department of Chemistry and Biochemistry
Class III HDACs (SIRTs) have
been receiving global attention as anticancer target molecules. Currently, a
dozen of classical HDAC inhibitors (classes I and II HDACs) are being evaluated
as anticancer drugs in clinical trials. In 2006, one of the HDAC inhibitors,
SAHA (Zolinza®), was approved as a drug for the treatment of cutaneous T-cell
lymphoma (CTCL) by the FDA. Although SIRT inhibitors have great potential to be
a new class of anticancer drugs, no potent SIRT inhibitors have been discovered
from natural resources. The research focuses on: (1) indentifying
marine-derived actinomycetes that produce SIRT inhibitors using yeast assay,
and (2) dereplication of active-strain crude extracts. A crude extract library
was developed from 70 different actinomycetes separated from deepwater and
shallow-water marine sediments. This chemical library was applied to a yeast
strain with Ura3 reporter gene in the telomere region. Active strains were
screened for selective toxicity in media containing 5-Fluorooritic acid (5-FOA)
against media without 5-FOA; in the presence of active strains, 5-FOA would
consequently be converted into 5-Fluorouracil (5-FU), a toxic compound. This
yeast assay provided several active strains. In this presentation, the results
obtained from the yeast screening and dereplication of active hits by LCMS
analysis will be shown.
San
Francisco State University, Department of Chemistry and Biochemistry
Epigenetic alterations
(i.e. those that do not involve changes in the underlying DNA sequencing) of
chromatin cause gene transcription and gene expression disorder connected to
tumorigenesis. Class I and II histone deacetylases (HDACs) are a group of
epigenetic enzymes. More than 10 classes of I and II HDAC inhibitors are in
clinical trial for anticancer agents. In order to explore HDAC inhibitors with
new structural motifs, a chemical library was screened prepared from 70 marine
sediments-derived actinomycetes against a genetically modified yeast strain.
This yeast strain possesses a reporter gene (Ura3) that converts 5-fluoroorotic
acid (5-FOA) into the cytotoxic compound, 5-fluorouracil (5-FU), resulting in
cell death. Several active actinomycetes were visually observed for selective
activity in the media with 5-FOA (no growth = clear media) against in the media
without 5-FOA (growth = cloudy media). The crude extracts of the active strains
were dereplicated using a combination of LCMS analysis and database search. In
the poster presentation, we will present the yeast screening results as well as
potential HDAC inhibitors obtained by dereplication.
Sonoma State
University, Department of Chemistry
Recent studies show that
chromium (III) aids in weight loss, but consuming large amounts of Cr (III) is
also potentially dangerous. In the body,
there are proteins that bind to chromium, but the exact structure and make-up
of these proteins are unknown. However,
two chromium-binding proteins have been partially characterized in the
literature, and these studies have determined the amino acid composition and
molar masses. The goal of this project
is to investigate the binding of various ligands and Cr (III) using an
isothermal calorimeter. Preliminary
results of the ligand nitrilotriacetic acid (NTA) with Cr (III) will be
presented. Preliminary data from the NTA
trials will be used to determine experimental parameters for the binding of
biologically relevant ligands. NTA forms
coordination compounds with metal ions, such as Cr (III), and will demonstrate
possible mechanisms and binding schemes for peptide bonding. The calorimetric data will be used to yield
both the enthalpy of the reaction and the equilibrium constant(s).
References:
1. Visser, H. G. (2006).
Acta Cryst. E62, m3272-m3274.
2. Visser, H. G. (2007).
Acta Cryst. E63, m162-m164.

California State
University, Sacramento, Department of Chemistry
There are many illnesses
associated with the formation of amyloids, which are protein aggregates toxic
to cells. Apolipoprotein A-I (apoA-I) is the major protein of HDL ("good
cholesterol") which, in its normal function, exerts many beneficial
effects that reduce the risk for heart disease. A number of naturally occurring
amyloid variants of apoA-I have been identified. We previously characterized
the amyloid variant G26R. Here, we report on the solubility properties of the
amyloid variant of L178H. To analyze solubility, both wild-type (WT) and L178H
proteins were incubated over a period of one month at temperatures ranging from
4 to 37 °C. Aliquots were removed periodically and centrifuged to separate
insoluble aggregates from the soluble fraction and analyzed by SDS-PAGE. The
experiment was conducted at pH 7.2 and at 3.8 to examine the influence of acidic
pH on amyloid formation. Preliminary results indicate that WT protein remained
soluble when incubated at either 4 or 25 °C but L178H, though
soluble at 4 °C, became insoluble when incubated at 37 °C.
Insoluble aggregates appeared at day 4 and increased throughout the month-long
incubation period. These results confirm the amyloid propensity of L178H.
Mills College, Department of Chemistry
The ionic liquids
1-butyl-3-methylimidazolium bistriflamide and 1-butyl-2,3-dimethylimidazolium
bistriflamide were used as solvents for the solvolysis of adamantyl mesylate.
Investigations were made into doping the ionic liquid solvents with small
amounts of heteroatomic nucleophiles in an attempt to trap the carbocation
intermediates.
Santa
Clara University, Department of Chemistry and Biochemistry
We have synthesized three
primary amine submonomers for installation of a peptoid side chain
functionalized with 4-N,N-dimethylamino-1,8-naphthalimide (4-DMN). These
submonomers have varied chain length separating the naphthalimide core from the
peptoid backbone. We have included these 4-DMN functionalized side chains into
short peptoids, and have evaluated the peptoid fluorescence in solvents with a
range of dielectric constants. For all peptoids, the fluorescence emission is
highly responsive to solvent: the wavelength of maximum emission shifts, and
emission intensity is roughly 100 times greater in 1,4-dioxane than in aqueous
buffer.
Future studies will aim
to use 4-DMN modified peptoids to probe their tertiary structural features as
part of a long term goal to recapitulate complex biological structures with
abiological, sequence-specific polymers.
California State
University, Sacramento, Department of Chemistry
The naturally occurring
enediynes, including calicheamicin and esperamicin, intercalate and cleave
cellular DNA by formation of a 1,4-benzenoid diradical formed upon Bergman
cyclization of their enediyne unit. The ability of arenediynes to undergo
photo-Bergman cyclization offers an attractive method to increase specificity
of enediyne pro-drugs by selective light exposure. Numerous synthetic enediynes
derived from 1,2-bis(phenylethynyl)benzene have been demonstrated to undergo
photo-Bergman cyclization. These
substrates, however, typically require significant irradiation times at 300 nm
affording low yields of cyclic adducts.
We have prepared novel enetriyne and enetetrayne derivatives in an
effort to improve cyclization yields and potentially extend the wavelength of
light empoyed in photo-Bergman cyclizations.
The synthesis of 1,2-bis(4-phenylbuta-1,3-diyn-1-yl)benzene and
1-ethynyl-2-(4-phenylbuta-1,3-diyn-1-yl)benzene, along with their solid state
reactivity, thermal solution reactivity, and ongoing photochemical studies will
be presented.
San
Jose State University, Department of Chemistry
Many of the actions of
1a,25-dihydroxyvitamin D3 [1,25(OH)2D3] are mediated by binding to the nuclear
vitamin D receptor (VDR). VDR is a
member of a superfamily of nuclear receptors that are ligand-dependent
transcription factors. Ligand binding
induces conformational changes in the VDR that enable the receptor to interact
with other coactivators to modulate gene transcription. In order to better characterize the binding
of the VDR to 1,25(OH)2D3 and to analogs of 1,25(OH)2D3, we have cloned the
cDNA for the human VDR and several variants into the expression plasmid,
pE-SUMOstar (Lifesensors). The
full-length wild-type receptor (1-427), an isomeric form of the receptor
(4-427), the C-terminal ligand binding domain of the receptor (118-427), and
gold binding protein (4-27) receptor fusion were cloned using polymerase chain
reaction (PCR) and subcloned into the BbsI and XbaI sites of pE-SUMOstar. The resulting plasmids will be transformed
into E. coli ER2566 cells, and protein expression will be induced. Western blot analysis will be used to confirm
the expression of target variants, and the purification of the proteins will be
achieved through metal affinity chromatography.
Once purified, the proteins will be used in microcalorimetry studies to
determine thermodynamic parameters of ligand binding to the proteins. This information will assist in the rational
drug design of vitamin D hormone analogs for therapeutic interventions.
Sonoma State
University, Department of Chemistry
Chromium(III) is a widely
used dietary supplement and strong experimental evidence exists to support the
use of chromium (III) for weight lost and relief of symptoms associated with
type II diabetes. However, the mode of action in which chromium aids in glucose
metabolism is not currently understood. There are two general hypotheses
regarding the functional role of chromium binding proteins in biology. First, chromium binding proteins are thought
to inhibit PTPase in the insulin glucose pathway in order to increase glucose
metabolism. Second, chromium binding proteins could be used for chromium
detoxification. If chromium does aid in glucose metabolism through an
endogenous mode, it would be likely to occur through the structure-functional
relationship of chromium binding proteins.
Such proteins have been reported in the literature but very little data
exists regarding the structure-functional relationship of chromium binding
proteins. We have isolated a novel protein that has a high affinity for
chromium. We suspect that this high affinity for chromium is directly related
to the primary structure of the protein. The first goal in our research is to
elucidate the primary, and secondary protein structure as well as explore the
two general hypotheses regarding the functional role of chromium in
biology.
California State
University, Sacramento, Department of Chemistry
Cytochrome P450
monooxygenase may catalyze a three-step oxidation of gerayl sub-unit producing
the intermediate isodomoic acid A in the biosynthesis of the marine neurotoxin
domoic acid by the diatom Pseudo-nitzschia spp.
Similar oxidation reactions participate in the synthesis of the plant
hormone gibberellin in Gibberella fujikuror (fungus) and Arabidopsis thaliana
(flowering plant). The synthesis of a
defense molecule, diterpene resin acid, in Pinus taeda (pine tree) also
utilizes a cytochorme P450 monooxygenase.
These enzymes were used as queries in a homology search against the
GenBank amino acid sequence database using Basic Local Alignment Tool
(BLAST). Forty eight homologous amino
acids sequences were recovered and used to perform a homology search against
nucleotide sequence in an EST library derived from Pseudo-nitzschia
multiseries. Eighteen sequences showed
homology with an E-value range of 0.01 to 1e-5.
These sequences were then used in a BLAST search against non-DA
producing diatoms, Thalassiosira pseudonana and Phaeodactylum tricornutum. All 18 sequences showed some homology to
non-DA diatoms genome sequences, suggesting these sequences my not encode
enzymes unique to DA biosynthesis.
California State
University, Sacramento, Department of Chemistry
Over the past twenty
years enediynes have been extensively studied since the discovery of the
enediyne antitumor antibiotics including calicheamicin and dynemincin. While
the natural products employ chemical activation of the enediyne, cyclic and
phenylethynyl arenediynes are well documented to undergo photochemical Bergman
cyclization. To examine the effect of extended conjugation on enediyne
reactivity we have studied a series of naphthyl-substituted arenediynes that
undergo C1-C6 or competing 2+2 cyclization. In the course
of these studies we observed that incorporation of electron donating methoxy
substituents on the naphthalene scaffold enhances the overall photochemical
reactivity of the enediyne unit. To further examine this effect we have
prepared a series of arylethynyl arenediynes containing
6-methoxynaphthalen-2-yl, 4-methoxynaphthalen-1-yl, 2-methoxynaphthalen-1-yl
groups appended to the enediyne alkynes. The photochemical reactivity of these
electron rich arylethynyl arenediynes will provide insights into the role of
electronic as well as steric effects on naphthyl-substituted enediyne reaction
pathways.
California State
University, Sacramento, Department of Chemistry
Apolipoprotein A-I
(Apo-AI) is one of the major apolipoproteins present in High Density
Lipoprotein (HDL) cholesterol. HDL is a “good” form of cholesterol due to its
ability to transport cholesterol out of cells, which mitigates plaque buildup.
ApoA-I also exerts cardio-protective anti-inflammatory and anti-oxidant
properties. Naturally occurring variants of Apo-AI have been associated with
amyloid deposits in a tissue specific manner. The mechanism of amyloid
formation and the basis of tissue deposition are unknown. Previously, we
characterized the variant G26R which acquires additional beta structure typical
of amyloid proteins. Here, we examine the binding of the beta-strand detecting
fluorescent dye Thioflavin T to the amyloid variant L178H in comparison to
wild-type (WT) protein. L178H and WT proteins were expressed in E. coli,
purified by nickel affinity chromatography, and then incubated at 37oC over a
two week period. Aliquots of protein were removed at different time intervals
and examined for binding to ThT. ThT bound to WT at a rate similar to what we
previously observed. However, ThT did not appear to bind to L178H over the same
time period, despite evidence of increased insolubility and fibril formation.
These results suggest L178H forms an amyloid structure distinct from that of
G26R. Differences in amyloid formation may underlie tissue-specific deposition.
San
Jose State University, Department of Chemistry
The purpose of this
research is to investigate the structural response of a model protein,
polylysine, to changes in its local environment. This, in turn, provides
insight into the factors that influence protein folding, including molecular
crowding and hydration effects. The method used is encapsulation of the protein
in the pores of a silica glass matrix using the sol-gel technique, in which the
glass is formed around the protein under relatively mild temperature and pH
conditions. In addition, the surface of the glass pores can be modified with
positive, negative and neutral hydrophilic groups to alter its interaction with
the peptide and surrounding water. The resulting glasses are not only permeable
to water and small solutes, but also optically transparent, allowing for
spectral analysis of the trapped biomolecules. Circular dichroism spectroscopy
is used to compare the structure of polylysine in different glasses and under
various solvent conditions.
Thus far, the most
interesting results have been obtained in a glass modified with just 2.5% of a
neutral hydrophilic reagent. The fact that polylysine is positively charged,
yet its structure is significantly altered by the presence of a neutral group,
suggests that hydration effects may dominate over direct electrostatic
interactions in this system.
The ratio of
diastereomeric products A and B in acylation of 2-substituted cyclohexanols was
found to depend dramatically on the nature of substituents and solvents.
Inversion of diastereoselectivity upon change of a solvent was found for
certain combinations of reactants and conditions.

San
Jose State University, Department of Biochemistry
The research from our lab
is important because some diseases such as Alzheimer’s are the result of a
protein folding incorrectly. Using Dr. Daryl Egger’s “Non-Interaction Model”
which means that solute effects on protein conformation may be mediated through
changes in the thermodynamic properties of water as opposed to the existing
model that infers a preferential interaction between the reacting species
(Ac-Trp-NH2) and the secondary solute (salt ions), we have been able to analyze
salt ions in the Hofmeister series.
Thus, to understand hydration as a driving force in protein folding, we
used tryptophan to mimic the protein’s backbone and analyzed the solute effects
using an Anton Paar DMA 5000 Density Meter to calculate solubility points.
Ac-Trp-NH2 was analyzed in a 2M TRIS control buffer and in 2M LiCl, TMAOAc, and
LiOAc. LiCl and TMAOAc yielded solubility data that is not consistent with our
hypothesis of the Non-Interaction Model.
California State
University, Sacramento, Department of Chemistry
Domoic acid (DA) is a
marine toxin produced by diatoms of the genus Pseudo-nitzschia. Identifying
genes encoding for DA biosynthetic enzymes allows for future cloning and
enzymatic assays to confirm gene function and to enable research directed
toward the molecular regulation of DA biosynthesis. Bioinformatic analysis was
performed to identify nucleotide sequences potentially encoding the enzyme
responsible for the double bond isomerization step in the postulated
biosynthetic pathway. Literature searches were performed to identify enzymes
that catalyze similar reactions which were then compared to the
Pseudo-nitzschia multiseries EST cDNA library using Basic Local Alignment
Search Tool (BLAST). The resultant sequence matches were compared to the
non-redundant protein database to ensure they did not encode a previously well
characterized protein not related to DA biosynthesis. They were also used as a
query against the genome of the non-domoic acid producing diatom, Thalassiosira
pseudonana, under the assumption that if present this protein would not be
involved in the production of DA. A homology of isopentenyl-diphosphate
delta-isomerase was identified that is uniquely found in the Pseudo-nitzschia
library that may encode the enzyme responsible for the isomerization of
isodomoic acid A.
California State
University, Sacramento, Department of Chemistry
Domoic acid (DA) is a
neurotoxin produced by marine diatoms in the genus Pseudo-nitzschia. Understanding the biosynthetic pathway of DA
may reveal regulation mechanisms important for future research in controlling
environmental DA levels. Bioinformatics
was used to identify candidate sequences from a Pseudo-nitzchia multiseries EST
cDNA library that may encode the enzyme responsible for the condensation of
geranyl diphosphate with glutamic acid in the proposed biosynthetic pathway of
DA. Literature searches provided the
amino acid sequences for proteins catalyzing reactions similar to the
condensation of geranyl diphosphate and glutamic acid. The sequences were compared to the
Pseudo-nitzchia multiseries EST cDNA library to identify homologous sequences
in the diatom. Homologous sequences were
then used as queries in a search of non redundant protein sequences using a
Basic Local Alignment Tool (BLAST) to confirm that a non-DA function had not
previously been characterized for a protein having a very closely related
sequence. The candidate nucleotide
sequences from the Pseudo-nitzchia multiseries EST cDNA library were also
compared to the genome sequence of the non domoic acid producing diatom
Thalassiosira pseudonana with the assumption that candidate sequences found in
this organism are not likely to have a role in the biosynthesis of DA.
University of San Francisco, Department of Chemistry
We have used UV-Vis
spectroscopy to characterize a novel form of metal-polymer binding in solution
which is driven by favorable Lewis-acid/Lewis-base second-coordination
interactions taking place between polyvinylpyrrolidone and
ruthenium(II)pentaamminepyridine in acetonitrile as solvent. The relatively strong Lewis base pyrrolidone
monomer units bring about a measureable redshift in the MLCT absorption band of
the (NH3)5Ru(II)pyridine(2+) complex when they hydrogen
bond to the Lewis-acidic ammine hydrogens.
By following the spectral changes carefully as a function of
metal:polymer ratio, we are able to discern a sequence of
spectroscopically-distinct chromophore environments which we believe reflects
the average number of metal-pyrrolidone contacts possible at a given metal
loading (the extent of the observed redshift increases as the number of pyrrolidone
monomer units available per each metal goes up). Surprisingly, the data suggest that at
maximum loading there may be as many as 18-20 metal complexes associated with a
single polymer chain containing only 45 (on-average) monomer units in its entire
length. This would imply a
highly-charged and probably very "rod-like" supramolecular assembly
in solution. Efforts underway to
quantitate the equilibrium binding constant will be described.
University of San Francisco, Department of Chemistry
Intervalence charge-transfer
(IT) transitions of mixed-valence complexes such as [(NH3)5RuII-L-RuIII(NH3)5]+5 (where L=4,4’-bipyridine, or
1,2-bisbipyridylethene, bpe) have been measured in water as a function of
various added electrolytes including simple alkali-metal halides.
The surprising result is
that there is a qualitative difference in the direction of the spectral shift
arising from the addition of different halide anions. Fluoride salts exhibit a spectral blue shift
in contrast to the red shift obtained for added chloride, bromide, and
iodide.
Even more surprising is
the fact that there is a small but systematic effect due to the cation of the
halide salt with more “structure-breaking” cations leading to lower IT band
energies in the order Cs+ < Rb+ < K+ ~ Na+
< Li+ .
Thus we find that the
position of the IT band in water for these decaammine mixed-valence ruthenium
dimers turns out to be a sensitive new probe of the surrounding water structure
as it is modified by the addition of various salts. Furthermore, the observed spectral shifts
correlate with the Jones-Dole viscometric “B coefficient” which measures ion
effects on water structure.
Taq polymerase is a
thermostable DNA polymerase named after the thermophilic bacterium Thermus
aquaticus. It is widely used in polymerase chain reaction (PCR). T. aquaticus
was isolated from hot springs
and Taq polymerase was identified as an enzyme able to withstand the thermal
cycling conditions required for rapid amplification of DNA. Taq's optimum
temperature for activity is 75-80°C, with a half-life of 9 minutes at 97.5°C.
In current research, we
used Taq polymerase to study the relationship between its structure and
function based on the hypothesis that primary structure (amino acid sequence)
codes for it’s folded 3D structure and in turn it’s biological function. If the
structure of protein is modified by substitutions of important amino acid
residues, our hypothesis is that its structure and function are most likely
altered.
To investigate this relationship,
25 single amino acid mutants of recombinant Taq polymerase gene were attempted
using side directed mutagenesis. 23 of these mutants were successfully
transformed into super competent cells. The wild type and all the mutant
proteins were expresses and purified using ion-exchange chromatography. To
observe the effect of mutation, we compare the melting behavior of the mutants
with that of wild type protein. The protein denaturant guanidium hydrochloride
was employed to generate denaturation profiles using circular dichroism
spectroscopy to follow protein stability. 8 mutants have been analyzed so far.
California State
University, Sacramento, Department of Chemistry
Dendrimers are
spherical-shaped macromolecules with terminal polyfunctional endpoints that
have been of increasing interest in the biomedical field since the latter part
of the 20th century. Sugars may be placed at the end of a dendrimer
to create a new category of macromolecules called glycodendrimers. The
synthesis of glycodendrimers can be partitioned into two categories: divergent
and convergent. The goal of this research is to manufacture ethereal hydrophilic
glycodendrimers using divergent synthesis with great efficiency,
reproducibility, and yield. This is achieved by comparing an array of synthetic
pathways and purification techniques that combine to give the greatest yield,
with brief turnaround periods. A β-lactosyl amine sugar was used to
terminate the glycodendrimer through a BOP coupling reaction.
San
Jose State University, Department of Chemistry
The purpose of this
research is to develop methodologies for the exploitation of carbon nanotube
thin films as chemical and electrochemical sensors. In this study we prepared thin films of
carbon nanotubes (CNT) by solution casting methods and then used a novel hybrid
electrochemical-conductometric method to measure the CNT film electrical
conductivity as a function of electrochemical bias and stimulus. The bias between the CNT film and the
contacting electrolyte and the bias between the opposite ends of the CNT film
(referred to as K1 and K2) were simultaneously
controlled – analogous to a transistor with an electrolyte gate. A promising motif for sensing involved a
cyclic voltammetric scan of K1 and K2 but with a 10 mVPP, 1 kHz AC signal
summed onto the K2 potential, and thus generating a trans-film potential that
was exploited, using a lock-in-amplifier detector, to monitor the CNT film
conductance during the CV scan. In order
to explore the chemical sensitivity of the CNT films in this setting, we
examined blank electrolyte, dissolved ferrocene, and ultra-thin films of novel
pyrene-modified iron(II)-terpyridine complex (Fe-tp-py) that binds to the CNT
surface. Our principal finding is that
this measurement method appears to discriminate between the CNT film
trans-conductance and the interfacial electrochemical impedance because the
iron(II)-iron(III) transition of the Fe-tp-py induced a clear change in the
putative CNT film conductance that is distinct from blank or ferrocene control.












